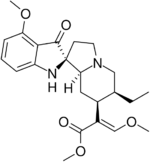
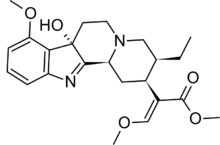
Short description: Chemical compound
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (2E)-2-[(2S,3S,7aS,12bS)-3-ethyl-7a-hydroxy-8-methoxy-1,2,3,4,6,7,7a,12b-octahydroindolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H30N2O5 | |
| Molar mass | 414.502 g·mol−1 |
| log P | 1.266 |
| Acidity (pKa) | 12.203 |
| Basicity (pKb) | 1.794 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as Kratom.[2] It is often referred to as ‘7-OH’. It was first described in 1994[3] and is a natural product derived from the mitragynine present in the Kratom leaf. It is considered an oxidized derivative and active metabolite of mitragynine.[4]
Metabolism
After a kratom study, it was revealed that 7-OH converts into Mitragynine pseudoindoxyl.[5]
| Compound | Affinities (Ki) | Ratio | Ref | ||
|---|---|---|---|---|---|
| MOR | DOR | KOR | MOR:DOR:KOR | ||
| 7-Hydroxymitragynine | 13.5 | 155 | 123 | 1:11:9 | [6] |
| Mitragynine | 7.24 | 60.3 | 1,100 | 1:8:152 | [6] |
| Mitragynine pseudoindoxyl | 0.087 | 3.02 | 79.4 | 1:35:913 | [6] |
See also
- Ajmalicine
- Mitragynine
- Mitragynine pseudoindoxyl
- Mitraphylline
- β-Prodine - molecule overlaying 7-hydroxymitragynine's opioid QSAR (Quantitative structure-activity relationship)
References
- ↑ 1.0 1.1 Chemical Abstracts Service: Columbus, OH, 2004; RN 174418-82-7 (accessed via SciFinder Scholar, version 2007.3; November 30, 2011)
- ↑ "Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa". Life Sciences 74 (17): 2143–55. March 2004. doi:10.1016/j.lfs.2003.09.054. PMID 14969718.
- ↑ Ponglux, Dhavadee; Wongseripipatana, Sumphan; Takayama, Hiromitsu; Kikuchi, Masae; Kurihara, Mika; Kitajima, Mariko; Aimi, Norio; Sakai, Shin-ichiro (1994). "A New Indole Alkaloid, 7 α-Hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand" (in en). Planta Medica 60 (6): 580–581. doi:10.1055/s-2006-959578. ISSN 0032-0943. PMID 17236085. https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-2006-959578.
- ↑ "7-Hydroxymitragynine - Green Leaf Kratom - Kratom Blogs Archives" (in en-US). 2020-08-19. https://www.greenleafkratom.com/7-hydroxymitragynine/.
- ↑ "Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2". J. Med. Chem. 59 (18): 8381–97. 2016. doi:10.1021/acs.jmedchem.6b00748. PMID 27556704.
- ↑ 6.0 6.1 6.2 "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. 2002. doi:10.1021/jm010576e. PMID 11960505.
External links
- "Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa" (pdf). Chemical & Pharmaceutical Bulletin 52 (8): 916–28. August 2004. doi:10.1248/cpb.52.916. PMID 15304982. https://www.jstage.jst.go.jp/article/cpb/52/8/52_8_916/_pdf. - synthesis of 7-hydroxymitragynine from mitragynine
 |