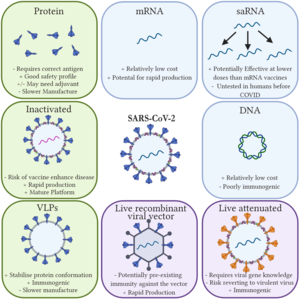
A ribonucleic acid (RNA) vaccine or messenger RNA (mRNA) vaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response.[1] The vaccine transfects molecules of synthetic RNA into immune cells, where the vaccine functions as mRNA, causing the cells to build foreign protein that would normally be produced by a pathogen (such as a virus) or by a cancer cell. These protein molecules stimulate an adaptive immune response which teaches the body to identify and destroy the corresponding pathogen or cancer cells.[1] The mRNA is delivered by a co-formulation of the RNA encapsulated in lipid nanoparticles which protect the RNA strands and help their absorption into the cells.[2][3]
Reactogenicity, the tendency of a vaccine to produce adverse reactions, is similar to that of conventional non-RNA vaccines.[4] People susceptible to an autoimmune response may have an adverse reaction to RNA vaccines.[4] The advantages of RNA vaccines over traditional protein vaccines are ease of design, speed and lower cost of production, and the induction of both cellular and humoral immunity.[5][6] RNA vaccines, such as the Pfizer–BioNTech COVID-19 vaccine, have the disadvantage of requiring ultracold storage before distribution;[1] other mRNA vaccines, such as the Moderna, CureVac, and Walvax COVID-19 vaccines, do not require such ultracold storage temperatures.[7][8]
In RNA therapeutics, mRNA vaccines have attracted considerable interest as COVID-19 vaccines.[1] In December 2020, Pfizer–BioNTech and Moderna obtained approval for their mRNA-based COVID-19 vaccines. On 2 December, the UK's Medicines and Healthcare products Regulatory Agency (MHRA) became the first medicines regulator to approve an mRNA vaccine, authorizing the Pfizer–BioNTech vaccine for widespread use.[9][10][11] On 11 December, the US Food and Drug Administration (FDA) issued an emergency use authorization for the Pfizer–BioNTech vaccine[12][13] and a week later similarly approved the Moderna vaccine.[14][15]
The use of RNA in vaccines has occasioned substantial misinformation in social media, wrongly claiming that the introduction of RNA alters a person's DNA.[16][17]
History
Early research
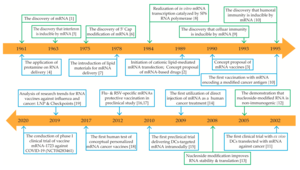
The first successful transfection of mRNA packaged within a liposomal nanoparticle into a cell was published in 1989.[18][19] "Naked" (or unprotected) mRNA was injected a year later into the muscle of mice.[3][20] These studies were the first evidence that in vitro transcribed mRNA could deliver the genetic information to produce proteins within living cell tissue[3] and led to the concept proposal of messenger RNA vaccines.[21][22]
Liposome-encapsulated mRNA was shown in 1993 to stimulate T-cells in mice,[23][24] and mRNA proved useful two years later to elicit both humoral and cellular immune response against a pathogen.[3][25][26]
Development
Successful application of modified nucleosides as a medium to get mRNA inside cells without setting off the body's defense system was reported in 2005.[3][27] The companies, BioNTech in 2008 and Moderna in 2010, were started to develop mRNA biotechnologies.[28][29]
US government agency DARPA launched in 2010 a biotech research program called ADEPT as part of its mission to develop emerging technologies for the US military.[30] DARPA recognized a year later the potential of nucleic acid technology for defense against pandemics and began to invest in the field through ADEPT.[30][31] DARPA's grants were seen as a vote of confidence which in turn encouraged other government agencies and private investors to also invest in mRNA technology.[31] In 2013, DARPA awarded a $25 million grant to Moderna.[32]
mRNA drugs for cardiovascular, metabolic and renal diseases, and selected targets for cancer were initially linked to serious side effects.[33][34] mRNA vaccines for human use have been studied for rabies, Zika virus disease, cytomegalovirus, and influenza.[35]
Acceleration
In December 2020, BioNTech and Moderna obtained approval for their mRNA-based COVID-19 vaccines. On 2 December, seven days after its final eight-week trial, the UK's Medicines and Healthcare products Regulatory Agency (MHRA), became the first global medicines regulator in history to approve an mRNA vaccine, granting emergency authorization for Pfizer–BioNTech's BNT162b2 COVID-19 vaccine for widespread use.[9][10][36] On 11 December, the FDA gave emergency use authorization for the Pfizer–BioNTech COVID-19 vaccine and a week later similar approval for the Moderna COVID-19 vaccine.[37]
Mechanism
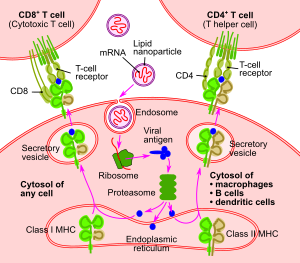
The goal of a vaccine is to stimulate the adaptive immune system to create antibodies that precisely target that particular pathogen. The markers on the pathogen that the antibodies target are called antigens.[38]
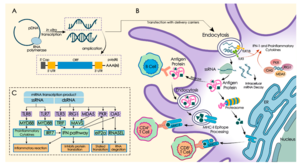
mRNA vaccines operate in a different manner from traditional vaccines.[1] Traditional vaccines stimulate an antibody response by injecting antigens, an attenuated virus (weakened virus), an inactivated virus (dead virus), or a recombinant antigen-encoding viral vector (harmless carrier virus with an antigen transgene) into the body. These antigens and viruses are prepared and grown outside the body.[39][40]
In contrast, mRNA vaccines introduce a short-lived[41] synthetically created fragment of the RNA sequence of a virus into the vaccinated individual. These mRNA fragments are taken up by dendritic cells – a type of immune system cell – by phagocytosis.[42] The dendritic cells use their internal machinery (ribosomes) to read the mRNA and produce the viral antigens that the mRNA encodes.[4] The body degrades the mRNA fragments within a few days.[43] Although non-immune cells can potentially also absorb vaccine mRNA, produce antigens, and display the antigens on their surfaces, dendritic cells absorb the mRNA globules much more readily.[44]
Once the viral antigens are produced by the host cell, the normal adaptive immune system processes are followed. Antigens are broken down by proteasomes, then class I and class II MHC molecules attach to the antigen and transport it to the cellular membrane, "activating" the dendritic cell.[45] Once the dendritic cells are activated, they migrate to lymph nodes, where the antigen is presented to T cells and B cells.[46] This eventually leads to the production of antibodies that are specifically targeted to the antigen, resulting in immunity.[38]
The benefit of using mRNA to have host cells produce the antigen is that mRNA is far easier for vaccine creators to produce than antigen proteins or attenuated virus.[47][1][4] Another benefit is speed of design and production. Moderna designed their mRNA-1273 vaccine for COVID-19 in 2 days.[48] Another advantage of RNA vaccines is that since the antigens are produced inside the cell, they stimulate cellular immunity, as well as humoral immunity.[6][49]
mRNA vaccines do not affect or reprogram DNA inside the cell. The synthetic mRNA fragment is a copy of the specific part of the viral RNA that carries the instructions to build the antigen of the virus (a protein spike, in the case of the main coronavirus mRNA vaccines), and is not related to human DNA. This misconception was circulated as the COVID-19 mRNA vaccines came to public prominence, and is considered a debunked conspiracy theory.[50][51]
Delivery
For a successful vaccine, enough mRNA has to enter the host cell cytoplasm to produce the specific antigens. The entry of mRNA molecules, however, faces a number of difficulties. mRNA molecules are too big to enter the cell membrane by simple diffusion. They are also negatively charged, like the cell membrane, which causes a mutual electrostatic repulsion. Additionally, mRNA is easily degraded by RNAases which exist in the skin and blood. Different methods have been developed to overcome these mRNA delivery hurdles. The method of vaccine delivery can be broadly classified by whether the mRNA transfer to cells happens within (in vivo) or outside (ex vivo) the organism.[52][3]
Ex vivo
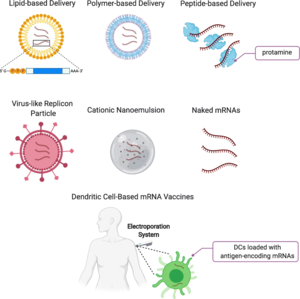
Dendritic cells are a type of immune cells that display antigens on their surfaces, leading to interactions with T cells to initiate an immune response. Dendritic cells can be collected from patients and programmed with the desired mRNA. Then, they can be re-administered back into patients to create an immune response.[53]
The simplest way that ex vivo dendtritc cells take up the mRNA molecules is through endocytosis. This is a fairly inefficient means of introducing mRNA into the cell in the laboratory setting and can be significantly improved by using electroporation. The ex vivo method allows mRNA to be introduced into cells with high efficiency.[52]
In vivo
Since the discovery that the direct administration of in vitro transcribed mRNA leads to the expression of antigens in the body, in vivo approaches have been investigated.[20] They offer some advantages over ex vivo methods, particularly by avoiding the cost of harvesting and adapting dendritic cells from patients, and by imitating a regular infection.[52]
Different routes of injection, such as into the skin, blood, or muscles, result in varying levels of mRNA uptake, making the choice of administration route a critical aspect of in vivo delivery. One study showed, in comparing different routes, that lymph node injection leads to the largest T-cell response.[54]
Naked mRNA injection
Naked mRNA injection means that the delivery of the vaccine is only done in a buffer solution.[55] This mode of mRNA uptake has been known since the 1990s.[20] The first worldwide clinical studies used intradermal injections of naked mRNA for vaccination.[56][57] A wide range of other injections methods have been used to deliver naked mRNA, such as subcutaneous injection, intravenous injection, and intratumoral injection. Although naked mRNA delivery causes an immune response, the effect is relatively weak, and after injection the mRNA is often rapidly degraded.[52]
Polymer and peptide vectors
Cationic polymers can be mixed with mRNA to generate protective coatings called polyplexes. These protect the recombinant mRNA from ribonucleases and assist its penetration in cells. Protamine is a natural cationic peptide and has been used to encapsulate mRNA for vaccination.[58][59]
Lipid nanoparticle vector
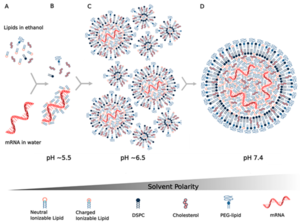
The first time the FDA approved the use of lipid nanoparticles as a drug delivery system was in 2018, when the agency approved the first siRNA drug, Onpattro.[60] Encapsulating the mRNA molecule in lipid nanoparticles was a critical breakthrough for producing viable mRNA vaccines, solving a number of key technical barriers in delivering the mRNA molecule into the host cell.[60][61] Research into using lipids to deliver siRNA to cells became a foundation for similar research into using lipids to deliver mRNA.[62] However, new lipids had to be invented to encapsulate mRNA strands, which are much longer than siRNA strands.[62]
Principally, the lipid provides a layer of protection against degradation, allowing more robust translational output. In addition, the customization of the lipid's outer layer allows the targeting of desired cell types through ligand interactions. However, many studies have also highlighted the difficulty of studying this type of delivery, demonstrating that there is an inconsistency between in vivo and in vitro applications of nanoparticles in terms of cellular intake.[63] The nanoparticles can be administered to the body and transported via multiple routes, such as intravenously or through the lymphatic system.[60]
One issue with lipid nanoparticles is that several of the breakthroughs leading to the practical use of that technology involve the use of microfluidics. Microfluidic reaction chambers are difficult to scale up, since the entire point of microfluidics is to exploit the microscale behaviors of liquids. The only way around this obstacle is to run an extensive number of microfluidic reaction chambers in parallel, a novel task requiring custom-built equipment.[64][65] For COVID-19 mRNA vaccines, this was the main manufacturing bottleneck. Pfizer used such a parallel approach to solve the scaling problem. After verifying that impingement jet mixers could not be directly scaled up,[66] Pfizer made about 100 of the little mixers (each about the size of a U.S. half-dollar coin), connected them together with pumps and filters with a "maze of piping,"[67][68] and set up a computer system to regulate flow and pressure through the mixers.[66]
Another issue, with the large-scale use of this delivery method, is the availability of the novel lipids used to create lipid nanoparticles, especially ionizable cationic lipids. Before 2020, such lipids were manufactured in small quantities measured in grams or kilograms, and they were used for medical research and a handful of drugs for rare conditions. As the safety and efficacy of RNA vaccines became clear by late 2020, the few companies able to manufacture the requisite lipids were confronted with the challenge of scaling up production to respond to orders for several tons of lipids.[65][69]
Viral vector
In addition to non-viral delivery methods, RNA viruses have been engineered to achieve similar immunological responses. Typical RNA viruses used as vectors include retroviruses, lentiviruses, alphaviruses and rhabdoviruses, each of which can differ in structure and function.[70] Clinical studies have utilized such viruses on a range of diseases in model animals such as mice, chicken and primates.[71][72][73]
Side effects and risks
Reactogenicity is similar to that of conventional, non-RNA vaccines. However, those susceptible to an autoimmune response may have an adverse reaction to RNA vaccines.[4] The mRNA strands in the vaccine may elicit an unintended immune reaction – this entails the body believing itself to be sick, and the person feeling as if they are as a result. To minimize this, mRNA sequences in mRNA vaccines are designed to mimic those produced by host cells.[5]
Strong but transient reactogenic effects were reported in trials of novel COVID-19 RNA vaccines; most people will not experience severe side effects which include fever and fatigue. Severe side effects are defined as those that prevent daily activity.[74]
General
Before 2020, no mRNA technology platform (drug or vaccine) had been authorized for use in humans, so there was a risk of unknown effects.[49] The 2020 COVID-19 pandemic required faster production capability of mRNA vaccines, made them attractive to national health organisations, and led to debate about the type of initial authorization mRNA vaccines should get (including emergency use authorization or expanded access authorization) after the eight-week period of post-final human trials.[75][76]
Storage
Because mRNA is fragile, some vaccines must be kept at very low temperatures to avoid degrading and thus giving little effective immunity to the recipient. Pfizer–BioNTech's BNT162b2 mRNA vaccine has to be kept between −80 and −60 °C (−112 and −76 °F).[77][78] Moderna says their mRNA-1273 vaccine can be stored between −25 and −15 °C (−13 and 5 °F),[79] which is comparable to a home freezer,[78] and that it remains stable between 2 and 8 °C (36 and 46 °F) for up to 30 days.[79][80] In November 2020, Nature reported, "While it's possible that differences in LNP formulations or mRNA secondary structures could account for the thermostability differences [between Moderna and BioNtech], many experts suspect both vaccine products will ultimately prove to have similar storage requirements and shelf lives under various temperature conditions."[49] Several platforms are being studied that may allow storage at higher temperatures.[4]
Advantages
Traditional vaccines
RNA vaccines offer specific advantages over traditional vaccines.[5][4] Because RNA vaccines are not constructed from an active pathogen (or even an inactivated pathogen), they are non-infectious. In contrast, traditional vaccines require the production of pathogens, which, if done at high volumes, could increase the risks of localized outbreaks of the virus at the production facility.[5] RNA vaccines can be produced faster, more cheaply, and in a more standardized fashion (with fewer error rates in production), which can improve responsiveness to serious outbreaks.[4][5] For example, the Pfizer–BioNTech vaccine originally required 110 days to produce (before Pfizer began to optimize the manufacturing process to only 60 days), but this was still far faster than traditional flu and polio vaccines.[67] Within that larger timeframe, the actual production time is only about 22 days: two weeks for molecular cloning of DNA plasmids and purification of DNA, four days for DNA-to-RNA transcription and purification of mRNA, and four days to encapsulate mRNA in lipid nanoparticles followed by fill and finish.[81] The majority of the days needed for each production run are allocated to rigorous quality control at each stage.[67]
DNA vaccines
In addition to sharing the advantages of theoretical DNA vaccines over established traditional vaccines, RNA vaccines also have additional advantages over DNA vaccines. The mRNA is translated in the cytosol, so there is no need for the RNA to enter the cell nucleus, and the risk of being integrated into the host genome is averted.[3] Modified nucleosides (for example, pseudouridines, 2'-O-methylated nucleosides) can be incorporated to mRNA to suppress immune response stimulation to avoid immediate degradation and produce a more persistent effect through enhanced translation capacity.[82][83][84] The open reading frame (ORF) and untranslated regions (UTR) of mRNA can be optimized for different purposes (a process called sequence engineering of mRNA), for example through enriching the guanine-cytosine content or choosing specific UTRs known to increase translation.[85] An additional ORF coding for a replication mechanism can be added to amplify antigen translation and therefore immune response, decreasing the amount of starting material needed.[86][87]
Vaccine hesitancy
There is misinformation implying that mRNA vaccines could alter DNA in the nucleus.[16] mRNA in the cytosol is very rapidly degraded before it would have time to gain entry into the cell nucleus. (mRNA vaccines must be stored at very low temperature to prevent mRNA degradation.) Retrovirus can be single-stranded RNA (just as SARS-CoV-2 vaccine is single-stranded RNA) which enters the cell nucleus and uses reverse transcriptase to make DNA from the RNA in the cell nucleus. A retrovirus has mechanisms to be imported into the nucleus, but other mRNA lack these mechanisms. Once inside the nucleus, creation of DNA from RNA cannot occur without a primer, which accompanies a retrovirus, but which would not exist for other mRNA if placed in the nucleus.[88]
Efficacy of mRNA vaccines for COVID-19
It is unclear why the novel mRNA COVID-19 vaccines from Moderna and Pfizer–BioNTech have shown potential efficacy rates of 90 to 95 percent when the prior mRNA drug trials on pathogens other than COVID-19 were not so promising and had to be abandoned in the early phases of trials.[89] Physician-scientist Margaret Liu stated that it could be due to the "sheer volume of resources" that went into development, or that the vaccines might be "triggering a nonspecific inflammatory response to the mRNA that could be heightening its specific immune response, given that the modified nucleoside technique reduced inflammation but hasn't eliminated it completely", and that "this may also explain the intense reactions such as aches and fevers reported in some recipients of the mRNA SARS-CoV-2 vaccines". These reactions though severe were transient and another view is that they were believed to be a reaction to the lipid drug delivery molecules.[89]
Unlike DNA molecules, the mRNA molecule is a very fragile molecule that degrades within minutes in an exposed environment, and thus mRNA vaccines need to be transported and stored at very low temperatures.[90] Outside the cell, or its drug delivery system, the mRNA molecule is also quickly broken down by the host.[5]
Self-amplifying RNA (saRNA) vaccines
The two main categories of mRNA vaccines are non-amplifying (conventional, viral delivery) and molecular self-amplifiying mRNA (non-viral delivery).[91][92] Self-amplifying mRNA vaccines began development in the 1990s.[93][86]
Self-amplifying RNA (saRNA) is a technology similar to mRNA, except the saRNA produces multiple copies of itself in the cell before producing proteins like mRNA does.[91][92] This allows smaller quantities to be used and has other potential advantages.[94][95]
The mechanisms and consequently the evaluation of self-amplifying mRNA may be different, as self-amplifying mRNA is fundamentally different by being a much bigger molecule in size.[3] saRNA vaccines are being researched, including development of a malaria vaccine.[96]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Non-viral COVID-19 vaccine delivery systems". Advanced Drug Delivery Reviews 169: 137–51. December 2020. doi:10.1016/j.addr.2020.12.008. PMID 33340620.
- ↑ "Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery". Mol Ther 27 (4): 710–28. April 2019. doi:10.1016/j.ymthe.2019.02.012. PMID 30846391.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Three decades of messenger RNA vaccine development". Nano Today 28: 100766. October 2019. doi:10.1016/j.nantod.2019.100766. https://biblio.ugent.be/publication/8628303.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "mRNA vaccines – a new era in vaccinology". Nature Reviews. Drug Discovery 17 (4): 261–79. April 2018. doi:10.1038/nrd.2017.243. PMID 29326426.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 PHG Foundation (2019). "RNA vaccines: an introduction". https://www.phgfoundation.org/briefing/rna-vaccines.
- ↑ 6.0 6.1 "Introduction to RNA Vaccines". RNA Vaccines: Methods and Protocols. Methods in Molecular Biology. 1499. 2017. pp. 1–11. doi:10.1007/978-1-4939-6481-9_1. ISBN 978-1-4939-6479-6.
- ↑ "Addressing the Cold Reality of mRNA Vaccine Stability". Journal of Pharmaceutical Sciences 110 (3): 997–1001. March 2021. doi:10.1016/j.xphs.2020.12.006. PMID 33321139.
- ↑ "Mexico to start late-stage clinical trial for China's mRNA COVID-19 vaccine" (in en). 2021-05-11. https://www.reuters.com/business/healthcare-pharmaceuticals/mexico-start-phase-iii-clinical-trials-chinas-walvax-covid-vaccine-2021-05-11/.
- ↑ 9.0 9.1 "UK authorises Pfizer/BioNTech COVID-19 vaccine" (Press release). Department of Health and Social Care. 2 December 2020.
- ↑ 10.0 10.1 "UK approves Pfizer/BioNTech Covid vaccine for rollout next week". The Guardian. 2 December 2020. https://www.theguardian.com/society/2020/dec/02/pfizer-biontech-covid-vaccine-wins-licence-for-use-in-the-uk.
- ↑ "Conditions of Authorisation for Pfizer/BioNTech COVID-19 Vaccine". Medicines & Healthcare Products Regulatory Agency. 8 December 2020. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/conditions-of-authorisation-for-pfizerbiontech-covid-19-vaccine.
- ↑ "FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine". U.S. Food and Drug Administration (FDA) (Press release). 11 December 2020. Retrieved 6 February 2021.
- ↑ "The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine – United States, December 2020". MMWR Morb Mortal Wkly Rep 69 (50): 1922–24. December 2020. doi:10.15585/mmwr.mm6950e2. PMID 33332292. PMC 7745957. https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6950e2-H.pdf.
- ↑ "FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine". U.S. Food and Drug Administration (FDA) (Press release). 18 December 2020.
- ↑ "The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine – United States, December 2020". MMWR Morb Mortal Wkly Rep 69 (5152): 1653–56. January 2021. doi:10.15585/mmwr.mm695152e1. PMID 33382675. https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm695152e1-H.pdf.
- ↑ 16.0 16.1 "Vaccine rumours debunked: Microchips, 'altered DNA' and more". BBC. 2 December 2020. https://www.bbc.co.uk/news/54893437.
- ↑ "What are mRNA vaccines and how do they work?: MedlinePlus Genetics" (in en). https://medlineplus.gov/genetics/understanding/therapy/mrnavaccines/.
- ↑ "mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection". International Journal of Molecular Sciences 21 (18): 6582. September 2020. doi:10.3390/ijms21186582. PMID 32916818. "Initiation of cationic lipid-mediated mrna transfection; Concept proposal of mRNA-based drugs.".
- ↑ "Cationic liposome-mediated RNA transfection". Proceedings of the National Academy of Sciences of the United States of America 86 (16): 6077–81. August 1989. doi:10.1073/pnas.86.16.6077. PMID 2762315. Bibcode: 1989PNAS...86.6077M.
- ↑ 20.0 20.1 20.2 "Direct gene transfer into mouse muscle in vivo". Science 247 (4949 Pt 1): 1465–8. March 1990. doi:10.1126/science.1690918. PMID 1690918. Bibcode: 1990Sci...247.1465W.
- ↑ "After COVID-19 successes, researchers push to develop mRNA vaccines for other diseases". Nature. May 31, 2021. https://www.nature.com/articles/s41591-021-01393-8?proof=t).. "When the broad range of vaccines against COVID-19 were being tested in clinical trials, only a few experts expected the unproven technology of mRNA to be the star. Within 10 months, mRNA vaccines were both the first to be approved and the most effective. Although these are the first mRNA vaccines to be approved, the story of mRNA vaccines starts more than 30 years ago, with many bumps in the road along the way. In 1990, the late physician-scientist Jon Wolff and his University of Wisconsin colleagues injected mRNA into mice, which caused cells in the mice to produce the encoded proteins. In many ways, that work served as the first step toward making a vaccine from mRNA, but there was a long way to go—and there still is, for many applications."
- ↑ "mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection". International Journal of Molecular Sciences 21 (18): 6582. September 2020. doi:10.3390/ijms21186582. PMID 32916818. "Concept proposal of mRNA vaccines (1990)".
- ↑ "Messenger RNA-based vaccines". Expert Opinion on Biological Therapy 4 (8): 1285–94. August 2004. doi:10.1517/14712598.4.8.1285. PMID 15268662.
- ↑ "Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA". European Journal of Immunology 23 (7): 1719–22. July 1993. doi:10.1002/eji.1830230749. PMID 8325342.
- ↑ "A development that may evolve into a revolution in medicine: mRNA as the basis for novel, nucleotide-based vaccines and drugs". Therapeutic Advances in Vaccines 2 (1): 10–31. January 2014. doi:10.1177/2051013613508729. PMID 24757523.
- ↑ "Characterization of a messenger RNA polynucleotide vaccine vector". Cancer Research 55 (7): 1397–400. April 1995. PMID 7882341. https://pubmed.ncbi.nlm.nih.gov/7882341/.
- ↑ "Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA" (in English). Immunity 23 (2): 165–75. August 2005. doi:10.1016/j.immuni.2005.06.008. PMID 16111635.
- ↑ "BioNTech's founders: scientist couple in global spotlight" (in en). 2020-11-13. https://www.france24.com/en/live-news/20201113-biontech-s-founders-scientist-couple-in-global-spotlight.
- ↑ "The story of mRNA: How a once-dismissed idea became a leading technology in the Covid vaccine race". 10 November 2020. https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/.
- ↑ 30.0 30.1 "How a secretive Pentagon agency seeded the ground for a rapid coronavirus cure". The Washington Post. July 30, 2020. https://www.washingtonpost.com/national-security/how-a-secretive-pentagon-agency-seeded-the-ground-for-a-rapid-coronavirus-cure/2020/07/30/ad1853c4-c778-11ea-a9d3-74640f25b953_story.html.
- ↑ 31.0 31.1 "DARPA's gambles might have created the best hopes for stopping COVID-19". BioCentury. March 19, 2020. https://www.biocentury.com/article/304691/darpa-s-gambles-might-have-created-the-best-hopes-for-stopping-covid-19.
- ↑ "DARPA Awards Moderna Therapeutics A Grant For Up To $25 Million To Develop Messenger RNA Therapeutics". 2 October 2013. https://www.prnewswire.com/news-releases/darpa-awards-moderna-therapeutics-a-grant-for-up-to-25-million-to-develop-messenger-rna-therapeutics-226115821.html.
- ↑ "Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine". 10 January 2017. https://www.statnews.com/2017/01/10/moderna-trouble-mrna/. "struggling to get mRNA into cells without triggering nasty side effects"
- ↑ "Ego, ambition, and turmoil: Inside one of biotech's most secretive startups". 13 September 2016. https://www.statnews.com/2016/09/13/moderna-therapeutics-biotech-mrna/. "because it’s exceedingly hard to get RNA into cells without triggering nasty side effects"
- ↑ "COVID-19 and Your Health" (in en-us). 11 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html.
- ↑ "Covid Pfizer vaccine approved for use next week in UK". BBC News. 2 December 2020. https://www.bbc.com/news/health-55145696.
- ↑ Office of the Commissioner (18 December 2020). "Pfizer-BioNTech COVID-19 Vaccine" (in en). https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- ↑ 38.0 38.1 "Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection". Advanced Drug Delivery Reviews 169: 168–89. December 2020. doi:10.1016/j.addr.2020.12.006. PMID 33316346.
- ↑ "SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates". NPJ Vaccines 6 (1): 28. February 2021. doi:10.1038/s41541-021-00292-w. PMID 33619260.
- ↑ "Recombinant vector vaccine evolution". PLOS Computational Biology 15 (7): e1006857. July 2019. doi:10.1371/journal.pcbi.1006857. PMID 31323032. Bibcode: 2019PLSCB..15E6857B.
- ↑ "Tools for translation: non-viral materials for therapeutic mRNA delivery". Nature Reviews Materials 2 (10): 17056. 12 September 2017. doi:10.1038/natrevmats.2017.56. Bibcode: 2017NatRM...217056H.
- ↑ "Developing mRNA-vaccine technologies". RNA Biology 9 (11): 1319–30. November 2012. doi:10.4161/rna.22269. PMID 23064118.
- ↑ "Review the safety of Covid-19 mRNA vaccines: a review". Patient Safety in Surgery 15 (1): 20. May 2021. doi:10.1186/s13037-021-00291-9. PMID 33933145.
- ↑ "How do the new COVID-19 vaccines work?". Scope. Stanford Medicine. December 22, 2020. https://scopeblog.stanford.edu/2020/12/22/how-do-the-new-covid-19-vaccines-work/.
- ↑ "mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection". International Journal of Molecular Sciences 21 (18): 6582. September 2020. doi:10.3390/ijms21186582. PMID 32916818.
- ↑ "mRNA Cancer Vaccines". Recent Results in Cancer Research. Fortschritte der Krebsforschung. Progres dans les Recherches Sur le Cancer. Recent Results in Cancer Research 209: 61–85. 2016. doi:10.1007/978-3-319-42934-2_5. ISBN 978-3-319-42932-8. PMID 28101688.
- ↑ "Seven vital questions about the RNA Covid-19 vaccines emerging from clinical trials". 19 November 2020. https://wellcome.org/news/seven-vital-questions-about-rna-covid-19-vaccines-pfizer-biontech-moderna.
- ↑ "Moderna's groundbreaking coronavirus vaccine was designed in just 2 days". 26 November 2020. https://www.businessinsider.com/moderna-designed-coronavirus-vaccine-in-2-days-2020-11?r=US&IR=T.
- ↑ 49.0 49.1 49.2 "COVID-19 vaccines poised for launch, but impact on pandemic unclear". Nature Biotechnology. November 2020. doi:10.1038/d41587-020-00022-y. PMID 33239758. https://www.nature.com/articles/d41587-020-00022-y.
- ↑ "Vaccine rumours debunked: Microchips, 'altered DNA' and more". 15 November 2020. https://www.bbc.com/news/54893437.
- ↑ "RNA Covid-19 vaccines will not change your DNA". 30 November 2020. https://fullfact.org/online/rna-vaccine-covid/.
- ↑ 52.0 52.1 52.2 52.3 "mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection". International Journal of Molecular Sciences 21 (18): 6582. September 2020. doi:10.3390/ijms21186582. PMID 32916818.
- ↑ "mRNA-based dendritic cell vaccines". Expert Review of Vaccines 14 (2): 161–76. February 2015. doi:10.1586/14760584.2014.957684. PMID 25196947.
- ↑ "Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity". Cancer Research 70 (22): 9031–40. November 2010. doi:10.1158/0008-5472.can-10-0699. PMID 21045153.
- ↑ "Vaccine components" (in en). 22 September 2016. https://www.immune.org.nz/vaccines/vaccine-development/vaccine-components.
- ↑ "Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent". Gene Therapy 14 (15): 1175–80. August 2007. doi:10.1038/sj.gt.3302964. PMID 17476302.
- ↑ "Protein expression from exogenous mRNA: uptake by receptor-mediated endocytosis and trafficking via the lysosomal pathway". RNA Biology 8 (4): 627–36. July 2011. doi:10.4161/rna.8.4.15394. PMID 21654214.
- ↑ Template:Primary-source inline"Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients". Journal of Immunotherapy 32 (5): 498–507. June 2009. doi:10.1097/CJI.0b013e3181a00068. PMID 19609242.
- ↑ "mRNA vaccine: a potential therapeutic strategy". Molecular Cancer 20 (1): 33. February 2021. doi:10.1186/s12943-021-01311-z. PMID 33593376.
- ↑ 60.0 60.1 60.2 "How nanotechnology helps mRNA Covid-19 vaccines work". 1 December 2020. https://www.statnews.com/2020/12/01/how-nanotechnology-helps-mrna-covid19-vaccines-work/.
- ↑ "mRNA vaccine delivery using lipid nanoparticles". Therapeutic Delivery 7 (5): 319–34. May 2016. doi:10.4155/tde-2016-0006. PMID 27075952.
- ↑ 62.0 62.1 "Without these lipid shells, there would be no mRNA vaccines for COVID-19". Chemical & Engineering News (American Chemical Society). March 6, 2021. https://cen.acs.org/pharmaceuticals/drug-delivery/Without-lipid-shells-mRNA-vaccines/99/i8.
- ↑ "A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation". Nano Letters 18 (3): 2148–57. March 2018. doi:10.1021/acs.nanolett.8b00432. PMID 29489381. Bibcode: 2018NanoL..18.2148P.
- ↑ "Opinion: A straightforward explanation why more COVID-19 vaccines can't be produced with help from 'dozens' of companies". MarketWatch. February 3, 2021. https://www.marketwatch.com/story/lets-stick-to-facts-about-covid-19-vaccines-there-arent-dozens-of-drug-companies-who-can-step-in-to-produce-more-11612363386.
- ↑ 65.0 65.1 "Why manufacturing Covid vaccines at scale is hard". Chemistry World (Royal Society of Chemistry). 23 March 2021. https://www.chemistryworld.com/news/why-manufacturing-covid-vaccines-at-scale-is-hard/4013429.article#/.
- ↑ 66.0 66.1 "Manufacturing moonshot: How Pfizer makes its millions of Covid-19 vaccine doses". CNN. April 2, 2021. https://www.cnn.com/2021/03/31/health/pfizer-vaccine-manufacturing/index.html.
- ↑ 67.0 67.1 67.2 "Race to the Vaccine: A COVID-19 vaccine life cycle: from DNA to doses". USA Today (Gannett). 7 February 2021. https://www.usatoday.com/in-depth/news/health/2021/02/07/how-covid-vaccine-made-step-step-journey-pfizer-dose/4371693001/.
- ↑ "mRNA Covid-19 Vaccines Are Fast to Make, but Hard to Scale". The Wall Street Journal. March 3, 2021. https://www.wsj.com/articles/mrna-covid-19-vaccines-are-fast-to-make-but-hard-to-scale-11614776401.
- ↑ "Why grandparents can't find vaccines: Scarcity of niche biotech ingredients". The Washington Post. February 18, 2021. https://www.washingtonpost.com/business/2021/02/18/vaccine-fat-lipids-supply/.
- ↑ "RNA Viruses as Tools in Gene Therapy and Vaccine Development". Genes 10 (3): 189. March 2019. doi:10.3390/genes10030189. PMID 30832256.
- ↑ "Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model". Human Gene Therapy 26 (2): 82–93. February 2015. doi:10.1089/hum.2014.100. PMID 25419577.
- ↑ "Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses". Virology 278 (1): 55–59. December 2000. doi:10.1006/viro.2000.0635. PMID 11112481.
- ↑ "Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections". The Journal of Infectious Diseases 204 (Suppl 3): S1075–81. November 2011. doi:10.1093/infdis/jir349. PMID 21987744.
- ↑ "Public needs to prep for vaccine side effects". Science 370 (6520): 1022. November 2020. doi:10.1126/science.370.6520.1022. PMID 33243869.
- ↑ "Experts Tell F.D.A. It Should Gather More Safety Data on Covid-19 Vaccines". New York Times. 22 October 2020. https://www.nytimes.com/2020/10/22/health/covid-vaccine-fda-advisory-committee.html.
- ↑ "Pfizer boss warns on risk of fast-tracking vaccines". Financial Times. 30 September 2020. https://www.ft.com/content/1a91c897-66d5-4bd5-ae9b-0b3be185dac8.
- ↑ "Pfizer-BioNTech COVID-19 Vaccine Vaccination Storage & Dry Ice Safety Handling". Pfizer. https://www.cvdvaccine-us.com/product-storage-and-dry-ice.
- ↑ 78.0 78.1 "Why Does Pfizer's COVID-19 Vaccine Need To Be Kept Colder Than Antarctica?". https://www.npr.org/sections/health-shots/2020/11/17/935563377/why-does-pfizers-covid-19-vaccine-need-to-be-kept-colder-than-antarctica.
- ↑ 79.0 79.1 "Fact Sheet for Healthcare Providers Administering Vaccine" (PDF). ModernaTX, Inc.. https://www.fda.gov/media/144637/download.
- ↑ "Moderna Announces Longer Shelf Life for its COVID-19 Vaccine Candidate at Refrigerated Temperatures" (in en). https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine.
- ↑ "From science to syringe: COVID-19 vaccines are miracles of science and supply chains". CTV News (Bell Media). February 27, 2021. https://www.ctvnews.ca/health/coronavirus/from-science-to-syringe-covid-19-vaccines-are-miracles-of-science-and-supply-chains-1.5327003.
- ↑ "Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA". Immunity 23 (2): 165–75. August 2005. doi:10.1016/j.immuni.2005.06.008. PMID 16111635.
- ↑ "Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA". Nucleic Acids Research 39 (21): e142. November 2011. doi:10.1093/nar/gkr695. PMID 21890902.
- ↑ "Nucleoside Modified mRNA Vaccines for Infectious Diseases". RNA Vaccines. Methods in Molecular Biology. 1499. Springer New York. 17 December 2016. pp. 109–21. doi:10.1007/978-1-4939-6481-9_6. ISBN 978-1-4939-6479-6.
- ↑ "Developing mRNA-vaccine technologies". RNA Biology 9 (11): 1319–30. November 2012. doi:10.4161/rna.22269. PMID 23064118.
- ↑ 86.0 86.1 "Enhancing immune responses using suicidal DNA vaccines". Nature Biotechnology 16 (6): 562–65. June 1998. doi:10.1038/nbt0698-562. PMID 9624688.
- ↑ "Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses". Molecular Therapy 26 (2): 446–55. February 2018. doi:10.1016/j.ymthe.2017.11.017. PMID 29275847.
- ↑ Skalka AM (2014). "Retroviral DNA Transposition: Themes and Variations". Microbiology Spectrum 2 (5): 1101–23. doi:10.1128/microbiolspec.MDNA3-0005-2014. ISBN 9781555819200. PMID 25844274.
- ↑ 89.0 89.1 "The Promise of mRNA Vaccines". The Scientist. 25 November 2020. https://www.the-scientist.com/news-opinion/the-promise-of-mrna-vaccines-68202. Retrieved 27 November 2020.
- ↑ "Could mRNA COVID-19 vaccines be dangerous in the long-term?". The Jerusalem Post. 17 November 2020. https://www.jpost.com/health-science/could-an-mrna-vaccine-be-dangerous-in-the-long-term-649253.
- ↑ 91.0 91.1 "Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines". Expert Opin Drug Deliv 11 (6): 885–99. June 2014. doi:10.1517/17425247.2014.901308. PMID 24665982.
- ↑ 92.0 92.1 "Nonviral delivery of self-amplifying RNA vaccines". Proc Natl Acad Sci U S A 109 (36): 14604–09. September 2012. doi:10.1073/pnas.1209367109. PMID 22908294. Bibcode: 2012PNAS..10914604G.
- ↑ "Self-replicating Semliki Forest virus RNA as recombinant vaccine". Vaccine 12 (16): 1510–14. December 1994. doi:10.1016/0264-410x(94)90074-4. PMID 7879415.
- ↑ "Self-amplifying RNA vaccines for infectious diseases". Gene Therapy 28 (3–4): 117–129. April 2021. doi:10.1038/s41434-020-00204-y. PMID 33093657.
- ↑ "saRNA Biology | About Self-Amplifying RNA Genome & How It Works". https://www.chimeron.com/sarna-biology/.
- ↑ "A Malaria Vaccine Candidate". 2021-03-01. https://blogs.sciencemag.org/pipeline/archives/2021/03/01/a-malaria-vaccine-candidate.
Further reading
- "mRNA-based therapeutics--developing a new class of drugs". Nat Rev Drug Discov 13 (10): 759–80. October 2014. doi:10.1038/nrd4278. PMID 25233993.
External links
- "Five things you need to know about: mRNA vaccines". Horizon. https://horizon-magazine.eu/article/five-things-you-need-know-about-mrna-vaccines.html.
- "RNA vaccines: an introduction". PHG Foundation. University of Cambridge. https://www.phgfoundation.org/briefing/rna-vaccines.
- "Understanding mRNA COVID-19 Vaccines". Centers for Disease Control and Prevention. 4 March 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html.
- "Understanding and Explaining mRNA COVID-19 Vaccines". 4 March 2021. https://www.cdc.gov/vaccines/covid-19/hcp/mrna-vaccine-basics.html.