 | |
| Names | |
|---|---|
| Trade names | Stromectol, Soolantra cream |
| |
| Clinical data | |
| Main uses | Scabies, river blindness[1] |
| Pregnancy category |
|
| Routes of use | By mouth, topical |
| Defined daily dose | 12 mg[2] |
| External links | |
| AHFS/Drugs.com | Systemic: Monograph Topical: Monograph |
| MedlinePlus | a607069 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 93% |
| Metabolism | Liver (CYP450) |
| Elimination half-life | 18 hours |
| Excretion | Feces; <1% urine |
| Chemical and physical data | |
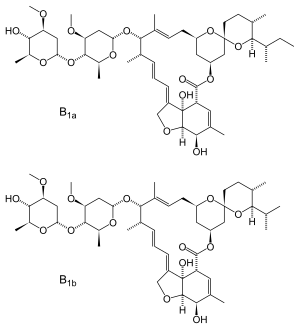
| Formula | C 48H 74O 14 (22,23-dihydroavermectin B1a) C 47H 72O 14 (22,23-dihydroavermectin B1b) |
| Molar mass | 875.106 g·mol−1 (22,23-dihydroavermectin B1a) 861.079 g·mol−1 (22,23-dihydroavermectin B1b) |
| 3D model (JSmol) | |
| |
| |
Ivermectin is a medication used to treat many types of parasite infestations.[3] This includes head lice, scabies, river blindness (onchocerciasis), strongyloidiasis, trichuriasis, ascariasis, and lymphatic filariasis.[3][4][5][6] It can be taken by mouth or applied to the skin for external infestations.[3][7] Use in the eyes should be avoided.[3]
Common side effects include red eyes, dry skin, and burning skin.[3] It is unclear if it is safe for use during pregnancy, but is probably acceptable for use during breastfeeding.[8] It belongs to the avermectin family of medications.[3] It works by causing the parasite's cell membrane to increase in permeability, resulting in paralysis and death.[3]
Ivermectin was discovered in 1975 and came into medical use in 1981.[9][10] It is on the World Health Organization's List of Essential Medicines.[11] The wholesale cost in the developing world for the tablets is about US$0.12 for a course of treatment.[12] In the United States, the costs is less than US$50 and is available over the counter.[13][14] In other animals, it is used to prevent and treat heartworm and acariasis, among other indications.[5]
Medical uses[edit | edit source]
Pinworms[edit | edit source]
Ivermectin is as effective as albendazole or alternative antinematode drugs for treatment of pinworm infection (enterobiasis).[15]
River blindness[edit | edit source]
Ivermectin is used for prevention, treatment, and control of river blindness (onchocerciasis) in populations where the disease is common. However, ivermectin is contraindicated in persons with a high burden of loiasis (e.g., >20,000 Loa loa microfilariae per mL), due to risk of ivermectin-associated severe inflammatory events.[16]
A single dose of ivermectin reduces microfilaridermia by 98–99% after 1–2 months.[17] Ivermectin does not kill adult worms. A single oral dose of ivermectin, taken once or twice a year for the 10–15-year lifespan of the adult worms, is required to protect the individual from river blindness.[18]
A 2012 Cochrane review found weak evidence suggesting that ivermectin could result in the reduction of chorioretinal lesions and prevent loss of vision in people with river blindness.[19] Moxidectin has been approved by the FDA for use in people with river blindness, has a longer half-life than ivermectin, and may eventually supplant ivermectin, as it is a more potent microfilaricide, but there is a need for additional clinical trials, with long-term follow-up, to assess whether moxidectin is safe and effective for treatment of nematode infection in children and women of childbearing potential.[20][21]
Loa loa[edit | edit source]
A single dose of ivermectin gives a rapid and durable decrease in body burden of eyeworm (Loa loa). The risk of ivermectin-associated severe adverse drug events is very low in persons with less than 20,000 microfilariae per mL of blood.[23]
Threadworm[edit | edit source]
Ivermectin is more effective than albendazole and equally as effective as thiabendazole for treatment of threadworm (enterobiasis). Ivermectin has fewer adverse effects than does thiabendazole and is at least as well tolerated as albendazole.[24] An analysis based on an economic model suggests that it is cost effective for people moving to Europe from areas where threadworm is common to be given a single-dose of ivermectin on arrival so as to cure presumptive infection with threadworm.[25] Persons who are immunocompromised or who will receive immunosuppressive treatment and who have confirmed or presumptive threadworm infestation are likely to benefit from treatment with ivermectin.[26]
Whipworm[edit | edit source]
Combination therapy with ivermectin plus albendazole is effective for treatment of whipworm (Trichuris trichiura) and the rate of Mazzotti reaction is no higher than for albendazole alone.[27]
Lymphatic filariasis[edit | edit source]
Combination therapy with ivermectin plus albendazole is effective for treatment of Lymphatic filariasis due to Wuchereria bancrofti,[28] Brugia malayi, or Brugia timori.[29]
Arthropod[edit | edit source]
Evidence supports its use against parasitic arthropods and insects:
- Mites such as scabies:[30][31][32] It is usually limited to cases that prove to be resistant to topical treatments or that present in an advanced state (such as Norwegian scabies).[32] One review found that the efficacy of permethrin is similar to that of systemic or topical ivermectin.[33] A separate review found that although oral ivermectin is usually effective for treatment of scabies, it does have a higher treatment failure rate than topical permethrin.[34] Another review found that oral ivermectin provided a reasonable balance between efficacy and safety.[35] Since ivermectin is more convenient than permethrin,[36] many have turned to veterinary sources of the drug to obtain assurance of a cure at an affordable price.[37]
- Lice:[38][39] Ivermectin lotion (0.5%) is FDA-approved for patients six months of age and older.[40] After a single, 10-minute application of this formulation on dry hair, 78% of subjects were found to be free of lice after two weeks.[41] This level of effectiveness is equivalent to other pediculicide treatments requiring two applications.[42]
- Bedbugs: There is tentative evidence that ivermectin kills bedbugs, as part of integrated pest management for bedbug infestations.[43][44][45] Such use however may require a prolonged course of treatment which is of unclear safety.[46]
- Malaria-bearing mosquitos, such as Anopheles gambiae: Mass drug administration of a population with ivermectin for purposes of treating/preventing nematode infestation is effective for eliminating malaria-bearing mosquitos and thereby reducing infection with residual malaria parasites.[47]
Rosacea[edit | edit source]
A review found that ivermectin was effective for treatment of rosacea.[48] An ivermectin cream has been approved by the FDA, as well as in Europe, for the treatment of inflammatory lesions of rosacea. The treatment is based upon the hypothesis that parasitic mites of the genus Demodex play a role in rosacea.[49] In a clinical study, ivermectin reduced lesions by 83% over 4 months, as compared to 74% under a metronidazole standard therapy.[50]
Dosage[edit | edit source]
The defined daily dose is 12 mg by mouth.[2] For scabies in those who weight over 15 kg a single dose of 0.2 mg/kg is recommended with the potential to repeat this dose after one week.[1] For river blindness in those over 15 kg 0.15 mg/kg is recommended with a second dose in three months if symptoms remain.[1] Further doses may be given at 6 to 12 month intervals.[1] It is not recommended in those who weight less than 15 kg.[1]
Side effects[edit | edit source]
The main concern is neurotoxicity, which in most mammalian species may manifest as central nervous system depression, and consequent ataxia, as might be expected from potentiation of inhibitory GABA-ergic synapses.
Since drugs that inhibit the enzyme CYP3A4 often also inhibit P-glycoprotein transport, the risk of increased absorption past the blood-brain barrier exists when ivermectin is administered along with other CYP3A4 inhibitors. These drugs include statins, HIV protease inhibitors, many calcium channel blockers, lidocaine, the benzodiazepines, and glucocorticoids such as dexamethasone.[51]
Ivermectin is contraindicated in children under the age of five or those who weigh less than 15 kilograms (33 pounds),[38] and individuals with liver or kidney disease.[52]
Pregnancy and breastfeeding[edit | edit source]
Ivermectin is secreted in very low concentration in breast milk.[53] It may be used in pregnancy.[1]
It remains unclear if ivermectin is safe during pregnancy and thus such use is not recommended.[54][1]
Pharmacology[edit | edit source]
Pharmacodynamics[edit | edit source]
Ivermectin and other avermectins (insecticides most frequently used in home-use ant baits) are macrocyclic lactones derived from the bacterium Streptomyces avermitilis. Ivermectin kills by interfering with nervous system and muscle function, in particular by enhancing inhibitory neurotransmission.
The drug binds to glutamate-gated chloride channels (GluCls) in the membranes of invertebrate nerve and muscle cells, causing increased permeability to chloride ions, resulting in cellular hyper-polarization, followed by paralysis and death.[3][55] GluCls are invertebrate-specific members of the Cys-loop family of ligand-gated ion channels present in neurons and myocytes.
Pharmacokinetics[edit | edit source]
Ivermectin can be given by mouth, topically, or via injection. It does not readily cross the blood–brain barrier of mammals due to the presence of P-glycoprotein,[56] (the MDR1 gene mutation affects function of this protein). Crossing may still become significant if ivermectin is given at high doses (in which case, brain levels peak 2–5 hours after administration). In contrast to mammals, ivermectin can cross the blood–brain barrier in tortoises, often with fatal consequences.
Ecotoxicity[edit | edit source]
Field studies have demonstrated the dung of animals treated with ivermectin supports a significantly reduced diversity of invertebrates, and the dung persists longer.[57]
History[edit | edit source]
The discovery of the avermectin family of compounds, from which ivermectin is chemically derived, was made by Satoshi Ōmura of Kitasato University, Tokyo and William C. Campbell of the Merck Institute for Therapeutic research. Ōmura identified avermectin from the bacterium Streptomyces avermitilis.[58] Campbell purified avermectin from cultures obtained from Ōmura and led efforts leading to the discovery of ivermectin, a derivative of greater potency and lower toxicity.[59] Ivermectin was introduced in 1981.[60] Half of the 2015 Nobel Prize in Physiology or Medicine was awarded jointly to Campbell and Ōmura for discovering avermectin, "the derivatives of which have radically lowered the incidence of river blindness and lymphatic filariasis, as well as showing efficacy against an expanding number of other parasitic diseases".[61]
Society and culture[edit | edit source]
Cost[edit | edit source]
The initial price, proposed by Merck in 1987, was US$6.[62] The company donated hundreds of millions of courses of treatments since 1988 in more than 30 countries.[62] Between 1995 and 2010 the program using donated ivermectin to prevent river blindness is estimated to have prevented 7 million years of disability well costing US$257 million.[63]
As of 2019[update], the cost effectiveness of treating scabies and lice with ivermectin has not been studied.[64][65]
As of 2019 ivermectin tablets in the United States were the least expensive treatment option for lice in children at about US$10.[66] The hair lotion, however, costs[which?] about US$300 for a course of treatment.[66]
Brand names[edit | edit source]
Ivermectin is available as a generic prescription drug in the U.S. in a 3 mg tablet formulation.[67] It is also sold under the brand names Heartgard, Sklice[68] and Stromectol[69] in the United States, Ivomec worldwide by Merial Animal Health, Mectizan in Canada by Merck, Iver-DT[70] in Nepal by Alive Pharmaceutical and Ivexterm in Mexico by Valeant Pharmaceuticals International. In Southeast Asian countries, it is marketed by Delta Pharma Ltd. under the trade name Scabo 6. The formulation for rosacea treatment is sold as Soolantra. While in development, it was assigned the code MK-933 by Merck.[71]
Veterinary use[edit | edit source]
Ivermectin is routinely used to control parasitic worms in the gastrointestinal tract of ruminant animals. These parasites normally enter the animal when it is grazing, pass the bowel and set and mature in the intestines, after which they produce eggs which leave the animal via its droppings and can infest new pastures. Ivermectin is effective in killing some, but not all, of these parasites.[citation needed]
In dogs it is routinely used as prophylaxis against heartworm.[72]
Dogs with defects in the P-glycoprotein gene (MDR1), often collie-like herding dogs, can be severely poisoned by ivermectin. The mnemonic "white feet, don't treat" refers to Scotch collies that are vulnerable to ivermectin.[73] Some other dog breeds (especially the Rough Collie, the Smooth Collie, the Shetland Sheepdog, and the Australian Shepherd), also have a high incidence of mutation within the MDR1 gene (coding for P-glycoprotein) and are sensitive to the toxic effects of ivermectin.[74][75] Clinical evidence suggests kittens are susceptible to ivermectin toxicity.[76] A 0.01% ivermectin topical preparation for treating ear mites in cats is available.[77]
Ivermectin is sometimes used as an acaricide in reptiles, both by injection and as a diluted spray. While this works well in some cases, care must be taken, as several species of reptiles are very sensitive to ivermectin. Use in turtles is particularly contraindicated.[78] For dogs, the insecticide spinosad may have the effect of increasing the toxicity of ivermectin.[79]
Research[edit | edit source]
Ivermectin is being studied as a potential antiviral agent against chikungunya and yellow fever.[80]
In 2013, this antiparasitic drug was demonstrated as a novel ligand of farnesoid X receptor (FXR),[81][82] a therapeutic target for Nonalcoholic Fatty Liver Disease.[83]
Ivermectin is also of interest in the prevention of malaria, as it is toxic to both the malaria plasmodium itself, and the mosquitos that carry it.[84][85]
COVID19[edit | edit source]
As of July 2021 it is unclear if ivermectin is effective or safe to treat or prevent COVID19.[86]
Ivermectin inhibits replication of SARS-CoV-2 in monkey kidney cell culture with an IC50 of 2.2 - 2.8 µM, making it a possible candidate for COVID-19 drug repurposing research.[87][88] The doses used in cell culture would require 104 larger doses in humans based on this data, which does not look promising as an effective treatment for COVID-19.[89][90] Such high doses of ivermectin are not covered by the current human-use approvals of the drug and could be dangerous, as the likely antiviral mechanism of action is the suppression of a host cellular process,[90] specifically the inhibition of nuclear transport by importin α/β1.[91]
On 10 April 2020, the FDA issued guidance to not use ivermectin intended for animals as treatment for COVID-19 in humans.[92]
See also[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "IVERMECTIN oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on August 28, 2021. Retrieved August 31, 2020.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on October 31, 2020. Retrieved August 31, 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Ivermectin". The American Society of Health-System Pharmacists. Archived from the original on January 3, 2016. Retrieved January 16, 2016.
- ↑ Sneader, Walter (2005). Drug Discovery a History. Chichester: John Wiley & Sons. p. 333. ISBN 978-0-470-01552-0. Archived from the original on June 15, 2020. Retrieved April 5, 2020.
- ↑ 5.0 5.1 Saunders Handbook of Veterinary Drugs: Small and Large Animal (4 ed.). Elsevier Health Sciences. 2015. p. 420. ISBN 978-0-323-24486-2. Archived from the original on January 31, 2016.
- ↑ CDC-Centers for Disease Control and Prevention (August 23, 2019). "Ascariasis - Resources for Health Professionals". www.cdc.gov. Archived from the original on November 21, 2010. Retrieved December 28, 2019.
- ↑ Panahi Y, Poursaleh Z, Goldust M (2015). "The efficacy of topical and oral ivermectin in the treatment of human scabies" (PDF). Annals of Parasitology. 61 (1): 11–16. PMID 25911032. Archived (PDF) from the original on April 4, 2020. Retrieved April 4, 2020.
- ↑ "Ivermectin Levels and Effects while Breastfeeding". Drugs.com. Archived from the original on January 1, 2016. Retrieved January 16, 2016.
- ↑ Mehlhorn, Heinz (2008). Encyclopedia of parasitology (3rd ed.). Berlin: Springer. p. 646. ISBN 978-3-540-48994-8. Archived from the original on January 31, 2016.
- ↑ Vercruysse J, Rew RS, eds. (2002). Macrocyclic lactones in antiparasitic therapy. Oxon, UK: CABI Pub. p. Preface. ISBN 978-0-85199-840-4. Archived from the original on January 31, 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Ivermectin". International Medical Products Price Guide. Archived from the original on April 5, 2020. Retrieved April 6, 2020.
- ↑ Commissioner, Office of the (October 27, 2020). "FDA Approves Lotion for Nonprescription Use to Treat Head Lice". FDA. Archived from the original on October 29, 2020. Retrieved October 30, 2020.
- ↑ "NADAC as of 2019-09-25 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on September 26, 2019. Retrieved September 26, 2019.
- ↑ Ottesen EA, Campbell WC (August 1994). "Ivermectin in human medicine". The Journal of Antimicrobial Chemotherapy. 34 (2): 195–203. doi:10.1093/jac/34.2.195. PMID 7814280.
- ↑ Keating J, Yukich JO, Mollenkopf S, Tediosi F (July 2014). "Lymphatic filariasis and onchocerciasis prevention, treatment, and control costs across diverse settings: a systematic review". Acta Tropica. 135: 86–95. doi:10.1016/j.actatropica.2014.03.017. PMID 24699086.
- ↑ Basáñez MG, Pion SD, Boakes E, Filipe JA, Churcher TS, Boussinesq M (May 2008). "Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 8 (5): 310–22. doi:10.1016/S1473-3099(08)70099-9. PMID 18471776. Archived from the original on August 29, 2021. Retrieved April 6, 2020.
- ↑ United Front Against Riverblindness. "Control of Riverblindness". Archived from the original on August 27, 2007.
- ↑ Ejere HO, Schwartz E, Wormald R, Evans JR (August 2012). "Ivermectin for onchocercal eye disease (river blindness)". The Cochrane Database of Systematic Reviews. 8 (8): CD002219. doi:10.1002/14651858.CD002219.pub2. PMC 4425412. PMID 22895928.
- ↑ Maheu-Giroux M, Joseph SA (August 2018). "Moxidectin for deworming: from trials to implementation". The Lancet. Infectious Diseases. 18 (8): 817–819. doi:10.1016/S1473-3099(18)30270-6. PMID 29858152.
- ↑ Boussinesq M (October 2018). "A new powerful drug to combat river blindness". Lancet. 392 (10154): 1170–1172. doi:10.1016/S0140-6736(18)30101-6. PMID 29361336.

- ↑ Huang, Yan-Ling; Liu, Lu; Liang, Hao; He, Jian; Chen, Jun; Liang, Qiao-Wen; Jiang, Zhi-Yuan; He, Jian-Feng; Huang, Min-Li; Du, Yi (January 24, 2020). "Orbital myiasis". Medicine. 99 (4): e18879. doi:10.1097/MD.0000000000018879. ISSN 0025-7974. Archived from the original on February 25, 2022. Retrieved February 24, 2022.
- ↑ Pion SD, Tchatchueng-Mbougua JB, Chesnais CB, Kamgno J, Gardon J, Chippaux JP, et al. (April 2019). "Loa loa Microfilaremia: Systematic Review and Meta-analysis". Open Forum Infectious Diseases. 6 (4): ofz019. doi:10.1093/ofid/ofz019. PMC 6449757. PMID 30968052.
- ↑ Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN (January 2016). "Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection". The Cochrane Database of Systematic Reviews (1): CD007745. doi:10.1002/14651858.CD007745.pub3. PMC 4916931. PMID 26778150.
- ↑ Agbata EN, Morton RL, Bisoffi Z, Bottieau E, Greenaway C, Biggs BA, Montero N, Tran A, Rowbotham N, Arevalo-Rodriguez I, Myran DT, Noori T, Alonso-Coello P, Pottie K, Requena-Méndez A (December 2018). "Effectiveness of Screening and Treatment Approaches for Schistosomiasis and Strongyloidiasis in Newly-Arrived Migrants from Endemic Countries in the EU/EEA: A Systematic Review". Int J Environ Res Public Health. 16 (1): 11. doi:10.3390/ijerph16010011. PMC 6339107. PMID 30577567.
- ↑ Kotton, Camille; Mileno, Maria; Keystone, J. (2019). "The Immunocompromised Traveler". Travel medicine. Edinburgh: Elsevier. pp. 269–277. ISBN 978-0-323-54696-6.
- ↑ Palmeirim MS, Hürlimann E, Knopp S, Speich B, Belizario V, Joseph SA, Vaillant M, Olliaro P, Keiser J (April 2018). "Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: A systematic review, meta-analysis and individual patient data analysis". PLOS Neglected Tropical Diseases. 12 (4): e0006458. doi:10.1371/journal.pntd.0006458. PMC 5942849. PMID 29702653.
- ↑ de Kraker ME, Stolk WA, van Oortmarssen GJ, Habbema JD (May 2006). "Model-based analysis of trial data: microfilaria and worm-productivity loss after diethylcarbamazine-albendazole or ivermectin-albendazole combination therapy against Wuchereria bancrofti". Tropical Medicine & International Health. 11 (5): 718–28. doi:10.1111/j.1365-3156.2006.01606.x. PMID 16640625.
- ↑ Taylor MJ, Hoerauf A, Bockarie M (October 2010). "Lymphatic filariasis and onchocerciasis". Lancet. 376 (9747): 1175–85. doi:10.1016/S0140-6736(10)60586-7. PMID 20739055. S2CID 29589578.
- ↑ Brooks PA, Grace RF (August 2002). "Ivermectin is better than benzyl benzoate for childhood scabies in developing countries". Journal of Paediatrics and Child Health. 38 (4): 401–4. doi:10.1046/j.1440-1754.2002.00015.x. PMID 12174005. S2CID 22499136.
- ↑ Victoria J, Trujillo R (2001). "Topical ivermectin: a new successful treatment for scabies". Pediatric Dermatology. 18 (1): 63–5. doi:10.1046/j.1525-1470.2001.018001063.x. PMID 11207977. Archived from the original on August 29, 2021. Retrieved April 6, 2020.
- ↑ 32.0 32.1 Strong M, Johnstone P (July 2007). Strong M (ed.). "Interventions for treating scabies". The Cochrane Database of Systematic Reviews (3): CD000320. doi:10.1002/14651858.CD000320.pub2. PMC 6532717. PMID 17636630.
- ↑ Rosumeck S, Nast A, Dressler C (April 2018). "Ivermectin and permethrin for treating scabies". Cochrane Database Syst Rev. 4: CD012994. doi:10.1002/14651858.CD012994. PMC 6494415. PMID 29608022.
- ↑ Dhana A, Yen H, Okhovat JP, Cho E, Keum N, Khumalo NP (January 2018). "Ivermectin versus permethrin in the treatment of scabies: A systematic review and meta-analysis of randomized controlled trials". Journal of the American Academy of Dermatology. 78 (1): 194–198. doi:10.1016/j.jaad.2017.09.006. PMID 29241784.
- ↑ Thadanipon K, Anothaisintawee T, Rattanasiri S, Thakkinstian A, Attia J (2019). "Efficacy and safety of antiscabietic agents: A systematic review and network meta-analysis of randomized controlled trials". J Am Acad Dermatol. 80 (5): 1435–1444. doi:10.1016/j.jaad.2019.01.004. PMID 30654070.

- ↑ Crump A, Ōmura S (February 10, 2011). "Ivermectin, 'wonder drug' from Japan: the human use perspective". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 87 (2): 13–28. Bibcode:2011PJAB...87...13C. doi:10.2183/pjab.87.13. PMC 3043740. PMID 21321478.
- ↑ Laing R, Gillan V, Devaney E (June 2017). "Ivermectin - Old Drug, New Tricks?". Trends in Parasitology. 33 (6): 463–472. doi:10.1016/j.pt.2017.02.004. PMC 5446326. PMID 28285851.

- ↑ 38.0 38.1 Dourmishev AL, Dourmishev LA, Schwartz RA (December 2005). "Ivermectin: pharmacology and application in dermatology". International Journal of Dermatology. 44 (12): 981–8. doi:10.1111/j.1365-4632.2004.02253.x. PMID 16409259. Archived from the original on August 29, 2021. Retrieved April 6, 2020.
- ↑ Strycharz JP, Yoon KS, Clark JM (January 2008). "A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae)". Journal of Medical Entomology. 45 (1): 75–81. doi:10.1603/0022-2585(2008)45[75:ANIFTK]2.0.CO;2. PMID 18283945.
- ↑ "Sklice lotion". drugs.com. Archived from the original on May 12, 2012.
- ↑ Pariser DM, Meinking TL, Bell M, Ryan WG (November 1, 2012). "Topical 0.5% ivermectin lotion for treatment of head lice". The New England Journal of Medicine. 367 (18): 1687–93. doi:10.1056/NEJMoa1200107. PMID 23113480.
- ↑ Healy, Melissa (November 1, 2012). "New treatment for lice is highly effective, study reports". Los Angeles Times. Archived from the original on January 1, 2020.
- ↑ Crump A (May 2017). "Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations". J. Antibiot. 70 (5): 495–505. doi:10.1038/ja.2017.11. PMID 28196978.
- ↑ Ōmura S (August 2016). "A Splendid Gift from the Earth: The Origins and Impact of the Avermectins (Nobel Lecture)". Angew. Chem. Int. Ed. Engl. 55 (35): 10190–209. doi:10.1002/anie.201602164. PMID 27435664. Archived from the original on August 2, 2020. Retrieved April 6, 2020.
- ↑ James, William D.; Elston, Dirk; Berger, Timothy; Neuhaus, Isaac (2015). Andrews' Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences. p. 439. ISBN 9780323319690. Archived from the original on June 26, 2020. Retrieved April 6, 2020.
Ivermectin treatment is emerging as a potential ancillary measure.
- ↑ Lebwohl, Mark G.; Heymann, Warren R.; Berth-Jones, John; Coulson, Ian (2017). Treatment of Skin Disease: Comprehensive Therapeutic Strategies. Elsevier Health Sciences. p. 89. ISBN 9780702069130. Archived from the original on June 26, 2020. Retrieved April 6, 2020.
- ↑ Tizifa TA, Kabaghe AN, McCann RS, van den Berg H, Van Vugt M, Phiri KS (2018). "Prevention Efforts for Malaria". Curr Trop Med Rep. 5 (1): 41–50. doi:10.1007/s40475-018-0133-y. PMC 5879044. PMID 29629252.
- ↑ Siddiqui K, Stein Gold L, Gill J (2016). "The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis". SpringerPlus. 5 (1): 1151. doi:10.1186/s40064-016-2819-8. PMC 4956638. PMID 27504249.
- ↑ Moran EM, Foley R, Powell FC (2017). "Demodex and rosacea revisited". Clin. Dermatol. 35 (2): 195–200. doi:10.1016/j.clindermatol.2016.10.014. PMID 28274359.
- ↑ "Galderma Receives FDA Approval of Soolantra (Ivermectin) Cream for Rosacea". drugs.com. Archived from the original on January 22, 2015."
- ↑ Goodman and Gilman's The Pharmacological Basis of Therapeutics Archived February 18, 2020, at the Wayback Machine, 11th edition, pages 122 Archived February 18, 2020, at the Wayback Machine, 1084–1087 Archived February 18, 2020, at the Wayback Machine.
- ↑ Heukelbach J, Winter B, Wilcke T, Muehlen M, Albrecht S, de Oliveira FA, Kerr-Pontes LR, Liesenfeld O, Feldmeier H (August 2004). "Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population". Bulletin of the World Health Organization. 82 (8): 563–71. PMC 2622929. PMID 15375445. Archived from the original on December 26, 2018. Retrieved December 26, 2018.
- ↑ Koh YP, Tian EA, Oon HH (September 2019). "New changes in pregnancy and lactation labelling: Review of dermatologic drugs". Int J Womens Dermatol. 5 (4): 216–226. doi:10.1016/j.ijwd.2019.05.002. PMC 6831768. PMID 31700976.
- ↑ Nicolas P, Maia MF, Bassat Q, Kobylinski KC, Monteiro W, Rabinovich NR, Menéndez C, Bardají A, Chaccour C (January 2020). "Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis". Lancet Glob Health. 8 (1): e92–e100. doi:10.1016/S2214-109X(19)30453-X. PMID 31839144.
- ↑ Yates DM, Wolstenholme AJ (August 2004). "An ivermectin-sensitive glutamate-gated chloride channel subunit from Dirofilaria immitis". International Journal for Parasitology. 34 (9): 1075–81. doi:10.1016/j.ijpara.2004.04.010. PMID 15313134.
- ↑ Borst P, Schinkel AH (June 1996). "What have we learnt thus far from mice with disrupted P-glycoprotein genes?". European Journal of Cancer. 32A (6): 985–90. doi:10.1016/0959-8049(96)00063-9. PMID 8763339.
- ↑ Iglesias LE, Saumell CA, Fernández AS, Fusé LA, Lifschitz AL, Rodríguez EM, Steffan PE, Fiel CA (December 2006). "Environmental impact of ivermectin excreted by cattle treated in autumn on dung fauna and degradation of faeces on pasture". Parasitology Research. 100 (1): 93–102. doi:10.1007/s00436-006-0240-x. PMID 16821034. S2CID 28765870.
- ↑ Saraiva RG, Dimopoulos G (March 2020). "Bacterial natural products in the fight against mosquito-transmitted tropical diseases". Natural Product Reports. 37 (3): 338–354. doi:10.1039/c9np00042a. PMID 31544193.
- ↑ Fisher MH, Mrozik H (1992). "The chemistry and pharmacology of avermectins". Annual Review of Pharmacology and Toxicology. 32: 537–53. doi:10.1146/annurev.pa.32.040192.002541. PMID 1605577.
- ↑ Campbell WC, Burg RW, Fisher MH, Dybas RA (June 26, 1984). "Chapter 1: The discovery of ivermectin and other avermectins". Pesticide Synthesis Through Rational Approaches. ACS Symposium Series. Vol. 255. American Chemical Society. pp. 5–20. doi:10.1021/bk-1984-0255.ch001. ISBN 978-0-8412-1083-7.
- ↑ "The Nobel Prize in Physiology or Medicine 2015" (PDF). Nobel Foundation. Archived from the original (PDF) on October 6, 2015. Retrieved October 7, 2015.
- ↑ 62.0 62.1 Crump A, Ōmura S (2011). "Ivermectin, 'wonder drug' from Japan: the human use perspective". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 87 (2): 13–28. Bibcode:2011PJAB...87...13C. doi:10.2183/pjab.87.13. PMC 3043740. PMID 21321478.
- ↑ Omaswa, Francis; Crisp, Nigel (2014). African Health Leaders: Making Change and Claiming the Future. OUP Oxford. p. PT158. ISBN 9780191008412. Archived from the original on August 3, 2020. Retrieved April 6, 2020.
- ↑ Chiu, Stephanie; Argaez, Charlene (2019). Ivermectin for Parasitic Skin Infections of Scabies: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines. CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. PMID 31424718. Archived from the original on August 28, 2021. Retrieved July 4, 2020.
- ↑ Young, Calvin; Argáez, Charlene (2019). Ivermectin for Parasitic Skin Infections of Lice: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines. CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. PMID 31487135. Archived from the original on August 28, 2021. Retrieved July 4, 2020.
- ↑ 66.0 66.1 Kliegman, Robert M.; St Geme, Joseph (2019). Nelson Textbook of Pediatrics E-Book. Elsevier Health Sciences. p. 3575. ISBN 9780323568883. Archived from the original on August 3, 2020. Retrieved April 6, 2020.
- ↑ U.S. FDA. "Abbreviated New Drug Application (ANDA): 204154". Drugs@FDA: FDA Approved Drug Products. U.S. Food and Drug Administration. Archived from the original on November 28, 2021. Retrieved August 18, 2018.
- ↑ "SKLICE- ivermectin lotion (NDC Code(s): 49281-183-71)". DailyMed. February 2012. Archived from the original on March 6, 2016. Retrieved September 9, 2015.
- ↑ "STROMECTOL- ivermectin tablet (NDC Code(s): 0006-0032-20)". DailyMed. May 2010. Archived from the original on March 6, 2016. Retrieved September 9, 2015.
- ↑ Adhikari, Santosh (May 27, 2014). "Alive Pharmaceutical (P) LTD.: Iver-DT". Alive Pharmaceutical (P) LTD. Archived from the original on March 4, 2016. Retrieved October 7, 2015.
- ↑ Pampiglione S, Majori G, Petrangeli G, Romi R (1985). "Avermectins, MK-933 and MK-936, for mosquito control". Transactions of the Royal Society of Tropical Medicine and Hygiene. 79 (6): 797–9. doi:10.1016/0035-9203(85)90121-X. PMID 3832491.
- ↑ Papich MG (January 1, 2016). "Ivermectin". In Papich MG (ed.). Saunders Handbook of Veterinary Drugs. Saunders Handbook of Veterinary Drugs (Fourth Edition). W.B. Saunders. pp. 420–423. doi:10.1016/B978-0-323-24485-5.00323-5. ISBN 978-0-323-24485-5. Archived from the original on April 7, 2020. Retrieved April 7, 2020.
- ↑ Dowling P (December 2006). "Pharmacogenetics: it's not just about ivermectin in collies". Can. Vet. J. 47 (12): 1165–8. PMC 1636591. PMID 17217086.
- ↑ "MDR1 FAQs". Australian Shepherd Health & Genetics Institute, Inc. Archived from the original on December 13, 2007.
- ↑ "Multidrug Sensitivity in Dogs". Washington State University's College of Veterinary Medicine. Archived from the original on June 23, 2015.
- ↑ Frischke H, Hunt L (April 1991). "Alberta. Suspected ivermectin toxicity in kittens". The Canadian Veterinary Journal. 32 (4): 245. PMC 1481314. PMID 17423775.
- ↑ "Acarexx". Boehringer Ingelheim. April 11, 2016. Archived from the original on February 17, 2019. Retrieved February 16, 2019.
- ↑ Klingenberg, Roger (2007). Understanding reptile parasites: from the experts at Advanced Vivarium Systems. Irvine, Calif: Advanced Vivarium Systems. ISBN 978-1882770908.
- ↑ "COMFORTIS® and ivermectin interaction Safety Warning Notification". U.S. Food and Drug Administration (FDA) Center for Veterinary Medicine (CVM). Archived from the original on August 29, 2009.
- ↑ Varghese FS, Kaukinen P, Gläsker S, Bespalov M, Hanski L, Wennerberg K, Kümmerer BM, Ahola T (February 2016). "Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses". Antiviral Research. 126: 117–24. doi:10.1016/j.antiviral.2015.12.012. PMID 26752081.
- ↑ Carotti A, Marinozzi M, Custodi C, Cerra B, Pellicciari R, Gioiello A, Macchiarulo A (2014). "Beyond bile acids: targeting Farnesoid X Receptor (FXR) with natural and synthetic ligands". Current Topics in Medicinal Chemistry. 14 (19): 2129–42. doi:10.2174/1568026614666141112094058. PMID 25388537.
- ↑ Jin L, Feng X, Rong H, Pan Z, Inaba Y, Qiu L, et al. (2013). "The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism". Nature Communications. 4: 1937. Bibcode:2013NatCo...4.1937J. doi:10.1038/ncomms2924. PMID 23728580.
- ↑ Kim SG, Kim BK, Kim K, Fang S (December 2016). "Bile Acid Nuclear Receptor Farnesoid X Receptor: Therapeutic Target for Nonalcoholic Fatty Liver Disease". Endocrinology and Metabolism. 31 (4): 500–504. doi:10.3803/EnM.2016.31.4.500. PMC 5195824. PMID 28029021.
- ↑ Chaccour C, Hammann F, Rabinovich NR (April 2017). "Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety". Malaria Journal. 16 (1): 161. doi:10.1186/s12936-017-1801-4. PMC 5402169. PMID 28434401.
- ↑ Siewe Fodjo JN, Kugler M, Hotterbeekx A, Hendy A, Van Geertruyden JP, Colebunders R (August 2019). "Would ivermectin for malaria control be beneficial in onchocerciasis-endemic regions?". Infectious Diseases of Poverty. 8 (1): 77. doi:10.1186/s40249-019-0588-7. PMC 6706915. PMID 31439040.
- ↑ Popp, Maria; Stegemann, Miriam; Metzendorf, Maria-Inti; Gould, Susan; Kranke, Peter; Meybohm, Patrick; Skoetz, Nicole; Weibel, Stephanie (July 28, 2021). "Ivermectin for preventing and treating COVID-19". Cochrane Database of Systematic Reviews. 2021 (7). doi:10.1002/14651858.cd015017.pub2. ISSN 1465-1858.
- ↑ Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (April 2020). "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro". Antiviral Research. 178: 104787. doi:10.1016/j.antiviral.2020.104787. PMC 7129059. PMID 32251768.

- ↑ Şimşek Yavuz S, Ünal S (April 2020). "Antiviral treatment of COVID-19". Turkish Journal of Medical Sciences. 50 (SI-1): 611–619. doi:10.3906/sag-2004-145. PMC 7195979. PMID 32293834.
- ↑ "TWiV 599: Coronavirus update - we need a plan | This Week in Virology". Archived from the original on April 25, 2020. Retrieved April 21, 2020.
- ↑ 90.0 90.1 Bray, Mike; Rayner, Craig; Noël, François; Jans, David; Wagstaff, Kylie (June 1, 2020). "Ivermectin and COVID-19: A report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors' responses". Antiviral Research. 178: 104805. doi:10.1016/j.antiviral.2020.104805. ISSN 0166-3542. PMC 7172803. PMID 32330482. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- ↑ Yang, Sundy N. Y.; Atkinson, Sarah C.; Wang, Chunxiao; Lee, Alexander; Bogoyevitch, Marie A.; Borg, Natalie A.; Jans, David A. (May 1, 2020). "The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer" (PDF). Antiviral Research. 177: 104760. doi:10.1016/j.antiviral.2020.104760. PMID 32135219. Archived (PDF) from the original on July 13, 2020. Retrieved July 9, 2020.
- ↑ "Do Not Use Ivermectin for Animals as Treatment for COVID-19 in Humans". U.S. Food and Drug Administration (FDA). April 10, 2020. Archived from the original on April 11, 2020. Retrieved April 10, 2020.
External links[edit | edit source]
| External sites: | |
|---|---|
| Identifiers: |
|
- The Carter Center River Blindness (Onchocerciasis) Control Program Archived September 17, 2020, at the Wayback Machine
- Trinity College Dublin. Prof William Campbell – The Story of Ivermectin Archived May 22, 2016, at the Wayback Machine
- "ivermectin (Rx) Stromectol". Medscape. Archived from the original on June 26, 2011. Retrieved April 6, 2020.
- "Ivermectin Topical". MedlinePlus. Archived from the original on May 1, 2017. Retrieved April 11, 2020.
.svg.png)
.svg.png)