 | |
| Names | |
|---|---|
| Trade names | Saflutan, Taflotan, Tapros, Zioptan, others |
| |
| Clinical data | |
| Drug class | Prostaglandin analogue[1] |
| Main uses | Open-angle glaucoma, ocular hypertension[1] |
| Side effects | Eye redness, itchiness, eyelash growth, blurry vision[1] |
| Pregnancy category |
|
| Routes of use | Topical (eye drops) |
| Onset of action | 2–4 hrs |
| Duration of action | ≥ 24 hrs |
| External links | |
| AHFS/Drugs.com | Multum Consumer Information |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Chemical and physical data | |
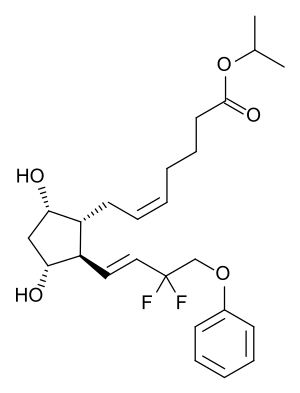
| Formula | C25H34F2O5 |
| Molar mass | 452.539 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tafluprost, sold under the brand name Zioptan among others, is a medication used to treat open-angle glaucoma and ocular hypertension.[1] It is used as an eye drop.[1] It may be used alone or with other medications.[1]
Common side effects include eye redness, itchiness, eyelash growth, and blurry vision.[1] Other side effects may include iritis and macular edema.[1] It is a prostaglandin analogue which is believed to work by increasing the outflow of aqueous fluid from the eye.[1]
Tafluprost was approved for medical use in the United States in 2012 and Canada in 2014.[1][2] In the United States it costs about 150 USD per month as of 2021.[3] In Canada it was less cost effective as compared to bimatoprost in 2020.[4]
Medical uses[edit | edit source]
It is used to treat open-angle glaucoma and ocular hypertension.[1]
Dosage[edit | edit source]
It is used as one drop once per day of a 0.0015% solution.[1]
Side effects[edit | edit source]
The most common side effect is conjunctival hyperemia, which occurs in 4 to 20% of patients. Less common side effects include stinging of the eyes, headache, and respiratory infections. Rare side effects are dyspnoea (breathing difficulties), worsening of asthma, and macular oedema.[5][6][7]
Interactions[edit | edit source]
Nonsteroidal anti-inflammatory drugs (NSAIDs) can either reduce or increase the effect of tafluprost.[5] Timolol eye drops, a common kind of glaucoma medication, does not negatively interact with this drug.[6]
No interactions with systemic (for example, oral) drugs are expected because tafluprost does not reach relevant concentrations in the bloodstream.[6][7]
Pharmacology[edit | edit source]
Mechanism of action[edit | edit source]
Tafluprost is a prodrug of the active substance, tafluprost acid, a structural and functional analogue of prostaglandin F2α (PGF2α). Tafluprost acid is a selective agonist at the prostaglandin F receptor, increasing outflow of aqueous fluid from the eyes and thus lowering intraocular pressure.[6][7]
Other PGF2α analogues with the same mechanism include latanoprost and travoprost.[6]
Pharmacokinetics[edit | edit source]
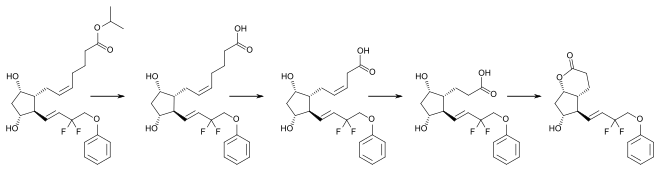
Tafluprost, as a lipophilic ester, easily penetrates the cornea and is then activated to the carboxylic acid, tafluprost acid. Onset of action is 2 to 4 hours after application, the maximal effect is reached after 12 hours, and ocular pressure remains lowered for at least 24 hours.[6][7]
Tafluprost acid is inactivated by beta oxidation to 1,2-dinortafluprost acid, 1,2,3,4-tetranortafluprost acid, and its lactone, which are subsequently glucuronidated or hydroxylated. The cytochrome P450 liver enzymes play no role in the metabolism.[7]
An analogous pathway (at least up to the tetranor-metabolites) has been found for latanoprost and travoprost.
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Tafluprost Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 20 September 2021.
- ↑ Canada, Health (2 July 2014). "Notice: Prescription Drug List (PDL): Multiple additions". www.canada.ca. Archived from the original on 8 July 2021. Retrieved 20 September 2021.
- ↑ "Tafluprost Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 1 May 2016. Retrieved 20 September 2021.
- ↑ "Prostaglandin Analogues for Ophthalmic Use: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines | CADTH". www.cadth.ca. Archived from the original on 22 September 2021. Retrieved 20 September 2021.
- ↑ 5.0 5.1 Tafluprost Professional Drug Facts.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ 7.0 7.1 7.2 7.3 7.4 Dinnendahl V, Fricke U, eds. (2011). Arzneistoff-Profile (in German). Vol. 9 (25 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Fukano Y, Kawazu K (August 2009). "Disposition and metabolism of a novel prostanoid antiglaucoma medication, tafluprost, following ocular administration to rats". Drug Metabolism and Disposition. 37 (8): 1622–34. doi:10.1124/dmd.108.024885. PMID 19477946. S2CID 12425702.
- ↑ Fukano Y, Kawazu K, Akaishi T, Bezwada P, Pellinen P (June 2011). "Metabolism and ocular tissue distribution of an antiglaucoma prostanoid, tafluprost, after ocular instillation to monkeys". Journal of Ocular Pharmacology and Therapeutics. 27 (3): 251–9. doi:10.1089/jop.2010.0178. PMID 21491995.
External links[edit | edit source]
| Identifiers: |
|
|---|