Oscillatory activity is an emerging property of the thalamocortical system. The various oscillatory rhythms generated in the thalamocortical system are mediated by two types of mechanisms:
- intrinsic mechanisms, which depend on the interplay between specific intrinsic currents.
- extrinsic or network mechanisms, which require the interaction of excitatory and inhibitory neurons within a population.
Intrinsic and network mechanisms can work alone (e.g., thalamic delta oscillations depend on the intrinsic properties of thalamic relay cells, cortical slow oscillation depends on network properties) or in combination (e.g., spindles depend on the interaction between thalamic relay and reticular neurons as well as on their intrinsic properties). The patterns and the dominant frequencies of thalamocortical oscillations depend on the functional state of the brain.
Contents |
[edit] Oscillations
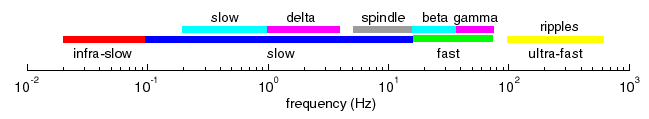
Normal thalamocortical oscillatory activities include
- infra-slow: 0.02-0.1 Hz,
- slow: 0.1-15 Hz (present mainly during slow-wave sleep or anesthesia), which are further divided on
- slow oscillation (0.2-1 Hz),
- delta (1-4 Hz),
- spindle (7-15 Hz),
- theta, which is generated in the limbic system and described elsewhere,
- fast: 20-60 Hz,
- ultra-fast: 100-600 Hz.
The fast and ultra-fast activities may be present in various states of vigilance including sleep and frequently coexist with slower rhythms (e.g., fast gamma oscillations may be found during depolarized phases of slow sleep oscillations). Spontaneous brain rhythms during different states of vigilance may lead to increased responsiveness and plastic changes in the strength of connections among neurons, thus affecting information flow in the thalamocortical system.
Each type of oscillation is generated by a particular set of intrinsic neuronal currents, synaptic interactions, and extracellular factors. Oscillations may also be generated in a population of non-pacemaker neurons coupled through gap junctions. Only "normal" thalamocortical activity is reviewed in this article; paroxysmal oscillations (such as seizures) are described elsewhere.
[edit] Infra-slow oscillation
This type of oscillatory activity has a period within the range of tens of seconds to a minute (Aladjalova, 1957). Very little is known about the underlying mechanisms of these oscillations but at least some of the factors responsible for their generation could depend on non-neuronal dynamics. Infra-slow activities likely have a cortical origin given that they can be recorded from small regions of neocortex devoid of their inputs by means of a surgical undercut (neocortical slabs; Aladjalova, 1962).
Functional role. Indirect evidence suggests that infra-slow oscillations synchronize faster activities, modulate cortical excitability, and contribute to the aggravation of epileptic activity during sleep (Vanhatalo et al., 2004).
[edit] Slow oscillation
During slow-wave sleep and some types of anesthesia the dominant activity pattern is slow oscillation, with frequency 0.3 - 1 Hz (Steriade et al., 1993a; Steriade et al., 2001). The following observations point to an intracortical origin for this rhythm:
- It survives extensive thalamic lesions in vivo (Steriade et al., 1993b) and exists in cortical in vitro preparations (Sanchez-Vives and McCormick, 2000).
- It is absent in the thalamus of decorticated cats (Timofeev and Steriade, 1996).
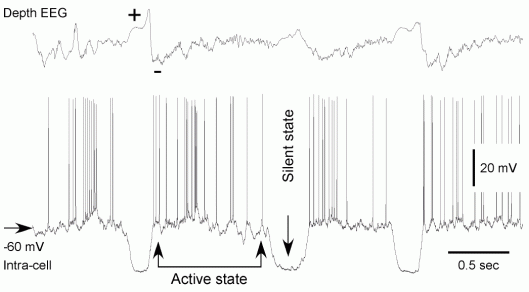
During slow oscillation the entire cortical network alternates between silent (Hyperpolarizing, or Down) and active (Depolarizing, or Up) states, each lasting 0.2-1 sec. At EEG level, the slow oscillation appears as periodic alterations of positive and negative waves (indicated by + and – signs in the figure).
- During EEG depth-positivity, cortical neurons remain in hyperpolarized, silent state.
- During EEG depth-negativity cortical neurons move to active states, reveal barrages of synaptic events and fire action potentials.
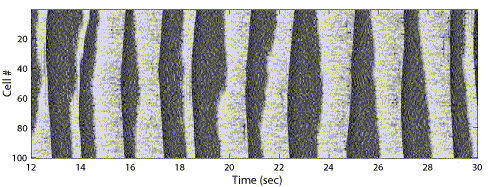
It was shown that during slow-wave sleep neocortical and thalamic neurons display phase relations that are restricted to narrow time windows (Contreras and Steriade, 1995). Recent studies suggest that the onsets of silent states are synchronized even better than the onsets of activity, and showed no latency bias for any location or cell type (Volgushev et al., 2006).
Intracellular studies on anesthetized and non-anesthetized cats have shown that the hyperpolarizing (DOWN) phase of the slow oscillation is associated with disfacilitation, a temporal absence of synaptic activity in all cortical and thalamic neurons (Timofeev et al. 1996; Timofeev et al. 2001a). Even a moderate spontaneous hyperpolarization of thalamic relay neurons during depth-positive EEG waves is sufficient to displace them from firing threshold, thereby affecting transmission of information toward the cerebral cortex (Timofeev et al. 1996). Responses to peripheral sensory stimuli still may reach the cerebral cortex during sleep or anesthesia, but the precision of cortical network to respond to peripheral volley during hyperpolarized (DOWN) periods is lost. The spike timing is critical in cortical information processing and therefore a minimal time interval of stable relay cells activity is required to achieve conscious perceptions (Libet et al. 1967). Thus, the conscious perception is impaired during sleep and anesthesia, likely, because the lost of precision in the sensory information transfer from periphery to the cerebral cortex.
At least two distinct mechanisms for the origin of slow cortical oscillations were proposed based on what causes the transition to the active (UP) state of the slow-sleep oscillation:
- Spontaneous miniature synaptic activities, or minis (Fatt and Katz, 1952), which are caused by the spike-independent release of transmitter vesicles and regulated at the level of single synapses. Occasional summation of the miniature EPSPs during the hyperpolarized (DOWN) phase of slow-sleep oscillations activates the persistent sodium current and depolarizes the membrane of cortical pyramidal cells, which is sufficient for spike generation (Timofeev et al., 2000; Bazhenov et al., 2002). This triggers the active phase, which propagates through the entire network and is maintained by synaptic activities and the persistent sodium current.
- Spontaneous activity of layer V cortical neurons (Sanchez-Vives and McCormick, 2000; Compte et al., 2003). It was shown, using a cortical slice preparation, that in relatively high concentrations (3.5 mM) of extracellular K+, cortical slices could oscillate in the frequency range of slow sleep oscillations (Sanchez-Vives and McCormick, 2000). This activity was usually initiated in layer V and propagated over the whole slice. A slight increase in extracellular K+ may depolarize some neurons to the firing threshold. In these conditions the relatively large amplitude EPSPs, but not minis, might recruit postsynaptic neurons into active states.
The total synaptic conductance progressively diminishes toward the end of active state in vivo (Contreras et al., 1996b). This suggests that active state termination is accompanied by a progressive run-down of synaptic activity. It supports either intrinsic mechanisms (build-up of a slow K+ conductance in single cells, reducing their firing) or synaptic mechanisms (build-up of a depressed state of excitatory synapses) for active state termination (Bazhenov et al., 2002). Either of these mechanisms may potentially explain refractoriness of the active states of slow-wave sleep found in slices (Sanchez and McCormick 2000). By contrast, in vivo, the waking state is associated with prolong depolarizing states, eventually lasting for the duration of the waking state. Thus, the refractoriness of an active state seems to be present in in vitro preparation only and could be attributed to the property of reduced network. Recent in vivo study revealed surprisingly high synchrony of active states termination (Volgushev et al., 2006) that implies the existence of a network mechanism which switches activity to silence.
A period and regularity of slow-wave sleep oscillations depend on the network size. While down states are relatively short in vivo (few hundreds msec), their duration can be tens of seconds in relatively small cortical slabs (Timofeev et al., 2000). It decreases approaching intact cortex in larger isolated gyrus preparations. This dependence on the network size was predicted by minis-based model of slow-wave sleep oscillation (Timofeev et al., 2000).
[edit] Delta oscillation
Field potential recordings from neocortex in human and animal models during sleep reveal the presence of delta oscillations (1-4 Hz). The delta oscillation likely has two different components, one of which originates in the neocortex and the other in the thalamus.
- Cortical delta activity. Both surgical removal of the thalamus and recordings from neocortical slabs in chronic conditions result in the significant enhancement of neocortical delta activity (Ball et al. 1977; Villablanca and Salinas-Zeballos 1972). Little is known about the cellular mechanisms mediating cortical delta oscillation. One of the hypotheses suggests that cortical delta could be driven by the discharge of intrinsically bursting neurons (Amzica and Steriade, 1998).
- Thalamic delta (1-4 Hz) is a well known example of rhythmic activity generated intrinsically by thalamic relay neurons as a result of the interplay between their low-threshold Ca2+ current (IT) and hyperpolarization activated cation current (Ih). As such, the delta oscillation may be observed during deep sleep when thalamic relay neurons are hyperpolarized sufficiently to deinactivate IT (McCormick and Pape, 1990). The mechanism of single cell delta activity is the following: a long-lasting hyperpolarization of thalamic relay neuron leads to slow Ih activation that depolarizes the membrane potential and triggers rebound burst, mediated by IT, which was deinactivated by the hyperpolarization. Both Ih (because of its voltage dependency) and IT (because of its transient nature) inactivate during burst, so membrane potential becomes hyperpolarized after burst termination. This after hyperpolarization starts next cycle of oscillations.
Periods of delta-like oscillation in thalamocortical neuron in decorticated cats can start from subtle fluctuations of the membrane potential. The amplitude of such activity increases and decreases without changes in frequency.
Synchrony between different thalamic relay neurons during delta activity has not been found in decorticated cats (Timofeev and Steriade 1996). Thus, it is unlikely that thalamic delta activity could play a leading role in the initiation and maintenance of cortical delta rhythm. However, the presence of a corticothalamic feedback in intact-cortex animals could synchronize thalamic burst-firing at delta frequency and generate field potentials.
At a certain level of leak current (Ileak), the ‘window’ component of IT in thalamocortical neurons, may create oscillations similar in frequency to the intrinsic thalamic delta oscillation (Williams et al., 1997).
Functional role of slow and delta oscillations. Slow wave sleep may be essential for memory consolidation and memory formation (Gais et al., 2000; Stickgold et al., 2000; Maquet, 2001; Huber et al., 2004). It has been proposed that synaptic plasticity associated with slow and delta oscillations could contribute to the consolidation of memory traces acquired during wakefulness (Steriade and Timofeev, 2003). Based on the analysis of multiple extracellular recordings of slow oscillations during natural sleep, it was suggested that fast oscillations during active states of slow-wave sleeps could reflect recalled events experienced previously; these events are "imprinted" in the network via synchronized network events that appear as slow-wave complexes in the EEG (Destexhe et al., 1997).
[edit] Sleep spindle oscillations
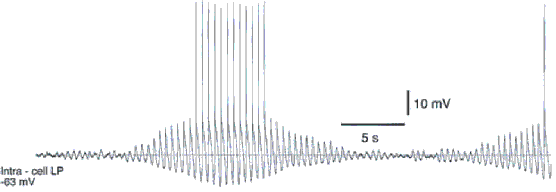
Sleep spindle oscillations consist of waxing-and-waning field potentials at 7-14 Hz, which last 1-3 seconds and recur every 5-15 seconds. In vivo, spindle oscillations are typically observed during the early stages of sleep or during active phases of slow-wave sleep oscillations.
In vivo, in vitro, and in silico studies suggest that the minimal substrate accounting for spindle oscillations consists in the interaction between thalamic reticular and relay cells (Steriade and Deschénes, 1984; Steriade et al., 1985; von Krosigk et al., 1993). Burst firing of reticular thalamic neurons induces inhibitory postsynaptic potentials in thalamocortical neurons. This deinactivates low-threshold Ca2+ current (IT), inducing burst firing in thalamocortical neurons which, in turn, excite reticular thalamic neurons allowing the cycle to start again. Spontaneous spindle oscillations are synchronized over large cortical areas during natural sleep and barbiturate anesthesia. After complete ipsilateral decortication, however, the long-range synchronization of thalamic spindles changes into disorganized patterns with low spatiotemporal coherence (Contreras et al., 1996).
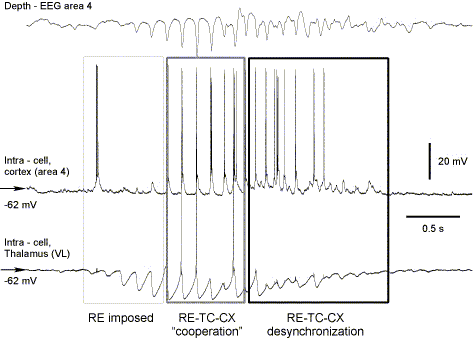
During spindle oscillations thalamocortical neurons do not fire every cycle of oscillations but intermit bursting with subthreshold oscillations. A simplest circuit model sufficient to generate this type of activity includes 2 reciprocally coupled reticular cells and 2 relay neurons providing excitation to and receiving inhibition from reticular neurons (Destexhe et al., 1996).
More complex models suggest the presence of at least three phases with different underlying mechanisms that contribute to the spindle generation.
- During the early phase of spindles, the reticular nucleus single-handedly drives the spindle oscillation via intrinsic mechanisms (Steriade et al., 1985). Several different mechanisms contributing to spindle generation in the isolated reticular nucleus were proposed:
- In the network simulations, thalamic reticular neurons organized with "dense proximal connectivity" generate spindle-like oscillations when are slightly depolarized (-60 to -70 mV) (Destexhe et al. 1994).
- Self-sustained spindle-like activity is generated in the model of isolated reticular nucleus when postsynaptic potentials between thalamic reticular cells reversed and became depolarizing at the relatively hyperpolarized membrane potentials that occur during sleep (Bazhenov et al. 1999).
- Gap junctions between thalamic reticular cells (Landisman et al., 2002) play an important role in the generation and synchronization of spindling activities in the thalamus (Fuentealba et al., 2004).
- The second component of spindles, on the other hand, primarily develops as a result of interactions between reticular and relay neurons (Destexhe et al., 1996; Bazhenov et al., 2000). Additionally, cortical firing contributes to spindle synchronization via cortico-thalamic neural firing, thereby imposing simultaneous excitation of reticular and relay neurons (Contreras et al., 1996). The role of cortical firing for spindle synchronization was studied in thalamocortical network models (Destexhe, et al., 1998, 1999). It was predicted that, in order to generate large-scale coherent oscillations, the cortex had to recruit the thalamus primarily through the RE nucleus. This result explains why propagating waves of spindle activity are found in vitro but not in vivo.
- The waning phase occurs as a result of network desynchronization (Timofeev et al., 2001) and of Ca2+ induced cAMP up-regulation of the hyperpolarization activated cation current, Ih, in relay cells (Bal and McCormick, 1996). Ca2+ mediated Ih activation tends to depolarize TC neurons preventing their rebound spike-bursts. This effect of Ih up-regulation was first predicted in the models (Destexhe et al., 1993) and later confirmed by in vitro experiments (Luthi & McCormick, 1998). When this up-regulation is prevented, spindles in slices do not wax-wane anymore but remain sustained (Luthi and McCormick, 1998). Ca2+ mediated Ih up-regulation can also explain refractoriness of spindle oscillations that was demonstrated in vitro (Kim et al., J Neurophysiol 1995) and in vivo (Contreras et al., J Neurosci 1997). The network desynchronization facilitates spindle termination in vivo and may have a few sources.
- The first is related to generation of rebound bursts in thalamic relay neurons with different delays from the onset of IPSP. The asynchronous burst firing of relay neurons will keep the membrane potential of thalamic reticular cells at relatively depolarized steady level, thus preventing the de-inactivation of IT and diminishing the probability of burst firing.
- Barrages of EPSPs from prethalamic relay stations (e.g. cerebellum) may produce a small but long-lasting depolarization, decrease input resistance of relay neurons and impair their bursting ability (Timofeev and Steriade, 1997) that could also desynchronize the thalamocortical network and disrupt the spindles.
- Because the trains of prethalamic EPSPs would occur only randomly, the most important source of spindle desynchronization, leading to decrease in their duration, is probably long-lasting spike-trains from neocortical neurons.
Functional role. Recent studies show that sleep related spindle oscillations are essential for memory formation (Gais et al., 2000) and demonstrate short- and middle term synaptic plasticity (reviewed by Steriade and Timofeev 2003). Spindling may activate the protein kinase A molecular "gate", thus opening the door for gene expression (Sejnowski and Destexhe, 2000) and allowing long-term changes to take place following subsequent inputs.
[edit] Beta-gamma oscillation
The waking state of the brain is characterized by the predominance of frequencies in the beta (15-30 Hz) and gamma (30-80 Hz) ranges (Bressler, 1990; Freeman, 1991). The fast rhythms are also synchronized between neighboring cortical sites during some forms of anesthesia, natural slow-wave, and REM sleep (Steriade et al., 1996a; Steriade et al., 1996b), when consciousness is either suspended or bizarre. During slow-wave sleep the fast rhythms follow the onset of depth-negative EEG wave.
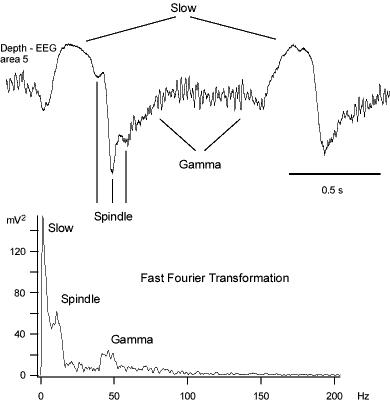
Gamma activity can exist in transient and persistent forms:
- Experimentally, transient (hundreds of milliseconds) gamma oscillations can be induced by tetanic stimulation of the hippocampus (Traub et al., 1996a). In this case, both fast spiking interneurons and pyramidal cells fire at the population frequency.
- Persistent gamma activity is found in CA3 (Fisahn et al., 1998) and neocortex (Buhl et al., 1998); this form of gamma can be induced by bath application of carbachol or kainate and the oscillations last minutes to hours. During persistent gamma activity interneurons fire on every cycle or every two cycles and pyramidal cells fire at lower frequencies.
Finally, it was found that GABAergic interactions in isolated interneuron networks may lead to network oscillation in the gamma frequency range (Traub et al., 1996b; Wang and Buzsaki, 1996). In both model and experiments it was shown that the frequency of these oscillations depends on the conductance and decay time of GABAA currents (Traub et al., 1996b). Large-scale network simulations revealed that coherent gamma range oscillations may appear through occasional increases in spiking synchrony within local groups of cortical neurons (Rulkov et al., 2004).
Origin of gamma oscillation. At least two non-exclusive basic mechanisms have been proposed to explain the origin of beta-gamma oscillations. One of them emphasizes extracortical and another one intracortical origin of these activities:
- A transient feed-forward synchronization to high-frequency peripheral (retinal, lemniscal or cerebellar) oscillations (Castelo-Branco et al. 1998; Timofeev and Steriade 1997) could impose the peripheral fast activities onto the thalamocortical system.
- Intracortical mechanisms themselves include several possibilities. The first one is based on the intrinsic property of fast rhythmic-bursting (FRB) neurons to fire fast spike-bursts at frequencies 20-60 Hz. These neurons were first described as fast pyramidal tract neurons from somatosensory cortex (Calvin and Sypert 1976), later they were found in layer II-III visual cortex (small pyramids called “chattering cells” (Gray and McCormick 1996)). The second intracortical mechanism of gamma activity generation depends on the activity of inhibitory interneurons and was described both in vitro and computational models (Borgers and Kopell 2003; Lytton and Sejnowski 1991; Traub et al. 1996a; Traub et al. 1997; Traub et al. 1998; Traub et al. 1999). Transitions between gamma and beta oscillations were simulated by alternating excitatory coupling between pyramidal neurons and by change in K+-conductances (Kopell et al. 2000; Traub et al. 1999). Lastly, role for gap junctions between axons of pyramidal cells in generating gamma oscillation was proposed (Traub et al. 2000). In this model spontaneous spiking activity in pyramidal cell axons was critical for persistent gamma oscillations. Transition from asynchronous network state to persistent gamma oscillations triggered by increase of pyramidal neurons excitability was later described in simplified network model with all-to-all connectivity (Borgers et al. 2005).
Gamma oscillations induced by visual stimuli can be synchronized over distances a few millimeters with near zero phase lag (Gray et al., 1989). Such precise synchronization in gamma frequency range was found between primary and associational visual cortexes (Engel et al., 1991; Frien et al., 1994) and between contralateral and parietal cortical areas (Desmedt and Tomberg, 1994). Synchronized gamma band activities were described in the visual cortex of anesthetized cats (Eckhorn et al., 1988; Gray et al., 1989) and awake monkeys (Kreiter and Singer, 1992). A number of experiments suggest that gamma-range synchronization in visual cortex may be restricted to few millimeters even with large coherent stimuli (large objects). Still the local features of these stimuli are perceived as coherently bound (Frien and Eckhorn, 2000). Propagating waves of gamma activity were described in primary visual cortex (Gabriel and Eckhorn, 2003) and the phase continuity of such gamma-waves (as opposite to strict long-rage synchrony) was proposed to be a basis of spatial feature binding across entire objects (Eckhorn et al., 2004).
Functional role. Gamma activity is associated with attentiveness (Rougeul-Buser et al., 1975; Bouyer et al., 1981), focused arousal (Sheer, 1989), sensory perception (Gray et al., 1989), movement (Murthy and Fetz, 1992; Pfurtscheller and Neuper, 1992) and prediction (Womelsdorf et al., 2006). It has been proposed that synchronization in the gamma frequency range is related to cognitive processing and important for temporal binding of sensory stimuli (Singer and Gray, 1995).
[edit] Ripples
Ultra-fast oscillations (>100 Hz), termed ripples, were described in CA1 hippocampal area and perirhinal cortex, where they were associated with bursts of sharp potentials during anesthesia, behavioral immobility, and natural sleep (Ylinen et al., 1995).
In the neocortex, ultra-fast oscillations (>200 Hz, up to 600 Hz) have been found
- in sensory-evoked potentials in rat barrel cortex (Jones and Barth, 1999; Jones et al., 2000),
- during high-voltage spike-and-wave patterns in rat (Kandel and Buzsáki, 1997). Neocortical networks seems to be sufficient to produce ripples as it was demonstrated in isolated cortical preparations (Grenier et al., 2001).
In addition to active inhibition (Ylinen et al., 1995; Grenier et al., 2001), the electrical coupling mediated by gap junctions contributes to the ripple synchronization (Draguhn et al., 1998; Grenier et al., 2003a). The electrical coupling may occur between axons of principal cells (Schmidt et al., 2001) or via a network of inhibitory interneurons (Galarreta and Hestrin, 1999; Gibson et al., 1999). Since ripples are recorded also in glial cells, the electrical coupling between glial cells could also play a role in the synchronization of ripples (Grenier et al., 2003a). The field potentials increase neuronal excitability, and by a positive feedback loop they could be also involved in the generation of neocortical ripples (Grenier et al., 2003b).
Functional role. Cortical ripples are generated during large amplitude spontaneous or evoked field potential deflections. These ample changes in the field potential are associated with synchronous activity of many neurons. This suggests that ripples may "alarm" the brain network about the presence of a large firing neuronal constellation. The danger of such a focal synchronous excitation of a neuronal pool is that it may overcome certain threshold of excitability, leading to the onset of seizures (Grenier et al., 2003b; Grenier et al., 2003a).
[edit] References
- Aladjalova NA (1957) Infra-slow rhythmic oscillations of the steady potential of the cerebral cortex. Nature 4567:957-959.
- Aladjalova NA (1962) Slow electrical processes in the brain. Moscow: Acad Sci USSA.
- Amzica F, Steriade M (1998) Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol 107:69-83.
- Bal T, McCormick DA (1996) What Stops Synchronized Thalamocortical Oscillations? Neuron 17:297-308.
- Ball GJ, Gloor P and Schaul N (1977) The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr Clin Neurophysiol 43: 346-361.
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ (1999) Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci 2:168-174.
- Bazhenov M, Timofeev I, Steriade M, Sejnowski T (2000) Patterns of spiking-bursting activity in the thalamic reticular nucleus initiate sequences of spindle oscillations in thalamic network. J Neurophysiol 84:1076-1087.
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ (2002) Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci 22:8691-8704.
- Borgers C and Kopell N (2003) Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput 15: 509-538.
- Borgers C, Epstein S, and Kopell NJ (2005) Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc Natl Acad Sci U S A 102: 7002-7007.
- Bouyer JJ, Montaron MF, Rougeul A (1981) Fast fronto-parietal rhythms during combined focused attentive behaviour and immobility in cat: cortical and thalamic localozations. Electroencephalography and Clinical Neurophsysiology 51:244-252.
- Bressler SL (1990) The gamma wave: a cortical information carrier? Trends Neurosci 13:161-162.
- Buhl EH, Tamas G, Fisahn A (1998) Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol (Lond) 513:117-126.
- Calvin WH, Sypert GW (1976) Fast and slow pyramidal tract neurons: an intracellular analysis of their contrasting repetitive firing properties in the cat. J Neurophysiol 39:420-434.
- Castelo-Branco M, Neuenschwander S, and Singer W (1998) Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci 18: 6395-6410.
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ (2003) Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol 89:2707-2725.
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M (1996) Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274:771-774.
- Contreras, D., Timofeev, I. and Steriade, M. (1996b). Mechanisms of long lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J. Physiol. 494, 251-264.
- Contreras D, Steriade M. (1995) Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15(1 Pt 2):604-22.
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M (1997) Spatiotemporal patterns of spindle oscillations in cortex and thalamus. J Neurosci. 17(3):1179-96.
- Desmedt JE, Tomberg C (1994) Transient phase-locking of 40 Hz electrical oscillations in prefrontal and parietal human cortex reflects the process of conscious somatic perception. Neurosci Lett 168:126-129.
- Destexhe, A., Babloyantz, A. and Sejnowski, T.J. (1993). Ionic mechanisms for intrinsic slow oscillations in thalamic relay neurons. Biophys. J. 65, 1538-1552.
- Destexhe A, Contreras D, Sejnowski TJ, Steriade M (1994) A model of spindle rhythmicity in the isolated thalamic reticular nucleus. J Neurophysiol 72:803-818.
- Destexhe A, Bal T, McCormick DA, Sejnowski TJ (1996) Ionic mechanisms underlaying synchronized and propagating waves in a model of ferret thalamic slices. J Neurophysiol 76:2049-2070.
- Destexhe A, Contreras D and Steriade M. (1998). Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J. Neurophysiol 79, 999-1016.
- Destexhe, A., Contreras, D. and Steriade, M. (1999). Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci. 19, 4595-4608.
- Draguhn A, Traub RD, Schmitz D, Jefferys JG (1998) Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature 394:189-192.
- Eckhorn R, Gail A, Bruns A, Gabriel A, Al-Shaikhli B, Saam M (2004) Neural mechanisms of visual associative processing. Acta Neurobiol Exp (Wars) 64:239-252.
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ (1988) Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern 60:121-130.
- Engel AK, Kreiter AK, Konig P, Singer W (1991) Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci U S A 88:6048-6052.
- Fatt P, Katz B (1952) Spontaneous sub-threshold activity at motor-nerve endings. Journal of Physiology 117:109-128.
- Fisahn A, Pike FG, Buhl EH, Paulsen O (1998) Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 394:186-189.
- Freeman WJ (1991) The physiology of perception. Sci Am 264:78-85.
- Frien A, Eckhorn R (2000) Functional coupling shows stronger stimulus dependency for fast oscillations than for low-frequency components in striate cortex of awake monkey. Eur J Neurosci 12:1466-1478.
- Frien A, Eckhorn R, Bauer R, Woelbern T, Kehr H (1994) Stimulus-specific fast oscillations at zero phase between visual areas V1 and V2 of awake monkey. Neuroreport 5:2273-2277.
- Fuentealba P, Crochet S, Timofeev I, Bazhenov M, Sejnowski TJ and Steriade M. (2004). Experimental evidence and modeling studies support a synchronizing role for electrical coupling in the cat thalamic reticular nucleus in vivo. Eur. J. Neurosci. 20, 111-119.
- Gabriel A, Eckhorn R (2003) A multi-channel correlation method detects traveling gamma-waves in monkey visual cortex. J Neurosci Methods 131:171-184.
- Gais S, Plihal W, Wagner U, Born J (2000) Early sleep triggers memory for early visual discrimination skills. Nat Neurosci 3:1335-1339.
- Galarreta M, Hestrin S (1999) A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402:72-75.
- Gibson JR, Beierlein M, Connors BW (1999) Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402:75-79.
- Gray CM, McCormick DA (1996) Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 274:109-113.
- Gray CM, Konig P, Engel AK, Singer W (1989) Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338:334-337.
- Grenier F, Timofeev I, Steriade M (2001) Focal synchronization of ripples (80-200 Hz) in neocortex and their neuronal correlates. J Neurophysiol 86:1884-1898.
- Grenier F, Timofeev I, Steriade M (2003a) Neocortical very fast oscillations (ripples, 80-200 Hz) during electrographic seizures: intracellular correlates. J Neurophysiol 89:841-852.
- Grenier F, Timofeev I, Crochet S, Steriade M (2003b) Spontaneous field potentials influence the activity of neocortical neurons during paroxysmal activities in vivo. Neuroscience 119:277-291.
- Huber R, Ghilardi MF, Massimini M, Tononi G (2004) Local sleep and learning. Nature 430:78-81.
- Jones MS, Barth DS (1999) Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol 82:1599-1609.
- Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS (2000) Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol 84:1505-1518.
- Kandel A, Buzsáki G (1997) Cellular-synaptic generation of sleep spindles, swpike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neuroscience 17:6783-6797.
- Kim U, Bal T and McCormick DA. (1995). Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. J. Neurophysiol. 74, 1301-1323.
- Kopell N, Ermentrout GB, Whittington MA, and Traub RD (2000) Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A 97: 1867-1872.
- Kreiter AK, Singer W (1992) Oscillatory Neuronal Responses in the Visual Cortex of the Awake Macaque Monkey. Eur J Neurosci 4:369-375.
- Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL and Connors BW. (2002)Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 22, 1002-1009.
- Libet B, Alberts WW, Wright EW, Jr. and Feinstein B (1967) Responses of human somatosensory cortex to stimuli below threshold for conscious sensation. Science 158: 1597-1600.
- Llinas RR, Grace AA, Yarom Y (1991) In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A 88:897-901.
- Luthi A and McCormick DA. (1998). Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron 20, 553-563.
- Lytton WW, Sejnowski TJ (1991) Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol 66:1059-1079.
- Maquet P (2001) The role of sleep in learning and memory. Science 294:1048-1052.
- McCormick DA, Pape HC (1990) Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol 431:319-342.
- Murthy VN, Fetz EE (1992) Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A 89:5670-5674.
- Pfurtscheller G, Neuper C (1992) Simultaneous EEG 10 Hz desynchronization and 40 Hz synchronization during finger movements. Neuroreport 3:1057-1060.
- Rougeul-Buser A, Bouyer JJ, Buser P (1975) From attentiveness to sleep. A topographical analysis of localized "synchronized" activities on the cortex of normal cat and monkey. Acta Neurobiol Exp (Warsz) 35:805-819.
- Rulkov NF, Timofeev I, Bazhenov M (2004) Oscillations in large-scale cortical networks: map-based model. J Comput Neurosci 17:203-223.
- Sanchez-Vives MV, McCormick DA (2000) Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3:1027-1034.
- Schmidt M, Sudkamp S, Wahle P (2001) Characterization of pretectal-nuclear-complex afferents to the pulvinar in the cat. Exp Brain Res 138:509-519.
- Sejnowski TJ, Destexhe A (2000) Why do we sleep? Brain Res 886:208-223.
- Sheer DE (1989) Focused arousal and the cognitive 40-Hz event-related potentials: differential diagnosis of Alzheimer's disease. Prog Clin Biol Res 317:79-94.
- Singer W, Gray CM (1995) Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 18:555-586.
- Steriade M, Deschénes M (1984) The thalamus as a neuronal oscillator. Brain Research Reviews 8:1-63.
- Steriade M, Timofeev I (2003) Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37:563-576.
- Steriade M, Nuñez A, Amzica F (1993a) A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13:3252-3265.
- Steriade M, Nuñez A, Amzica F (1993b) Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillations and other sleep rhythms of electroencephalogram. J Neurosci 13:3266-3283.
- Steriade M, Amzica F, Contreras D (1996a) Synchronization of fast (30-40 Hz) spontaneous cortical rhythms during brain activation. Journal of Neuroscience 16:392-417.
- Steriade M, Timofeev I, Grenier F (2001) Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85:1969-1985.
- Steriade M, Deschenes M, Domich L, Mulle C (1985) Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol 54:1473-1497.
- Steriade M, Contreras D, Amzica F, Timofeev I (1996b) Synchronization of fast (30-40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci 16:2788-2808.
- Stickgold R, James L, Hobson JA (2000) Visual discrimination learning requires sleep after training. Nat Neurosci 3:1237-1238.
- Timofeev I, Steriade M (1996) Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol 76:4152-4168.
- Timofeev I, Steriade M (1997) Fast (mainly 30-100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J Physiol 504 ( Pt 1):153-68.
- Timofeev I, Bazhenov M (2005) Mechanisms and biological role of thalamocortical oscillations. In: Trends in Chronobiology Research (Columbus F, ed), pp 1-47: Nova Science Publishers, Inc.
- Timofeev I, Bazhenov M, Sejnowski T, Steriade M (2001) Contribution of intrinsic and synaptic factors in the desynchronization of thalamic oscillatory activity. Thalamus and related systems 1:53-69.
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M (2000) Origin of slow cortical oscillations in deafferented cortical slabs. Cer Cortex 10:1185-1199.
- Traub RD, Whittington MA, Stanford IM, Jefferys JG (1996a) A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383:621-624.
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG (1996b) Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol 493 (Pt 2):471-484.
- Traub RD, Jefferys JG, and Whittington MA (1997) Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci 4: 141-150.
- Traub RD, Spruston N, Soltesz I, Konnerth A, Whittington MA, and Jefferys GR (1998) Gamma-frequency oscillations: a neuronal population phenomenon, regulated by synaptic and intrinsic cellular processes, and inducing synaptic plasticity. Prog Neurobiol 55: 563-575.
- Traub RD, Whittington MA, Buhl EH, Jefferys JG, and Faulkner HJ (1999) On the mechanism of the gamma --> beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J Neurosci 19: 1088-1105.
- Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, and Buhl EH (2000) A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci 12: 4093-4106.
- Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K (2004) Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A 101:5053-5057.
- Volgushev M, Chauvette S, Mukovski M, Timofeev I (2006) Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave sleep. J Neurosci, in press.
- Villablanca J and Salinas-Zeballos ME (1972) Sleep-wakefulness, EEG and behavioral studies of chronic cats without the thalamus: the 'athalamic' cat. Archives Italiennes de Biologie 110: 383-411.
- von Krosigk M, Bal T, McCormick DA (1993) Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261:361-364.
- Wang XJ, Buzsaki G (1996) Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16:6402-6413.
- Williams SR, Tóth TI, Turner JP, Hughes SW, Crunelli W (1997) The ‘window’ component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol 505:689-705.
- Womelsdorf T, Fries P, Mitra PP, Desimone R (2006) Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439:733-736.
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G (1995) Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15:30-46.
Internal references
- Eugene M. Izhikevich (2006) Bursting. Scholarpedia, 1(3):1300.
- Roger D. Traub (2006) Fast oscillations. Scholarpedia, 1(12):1764.
- Jeff Moehlis, Kresimir Josic, Eric T. Shea-Brown (2006) Periodic orbit. Scholarpedia, 1(7):1358.
- Philip Holmes and Eric T. Shea-Brown (2006) Stability. Scholarpedia, 1(10):1838.
- S. Murray Sherman (2006) Thalamus. Scholarpedia, 1(9):1583.