 | |
 | |
| Names | |
|---|---|
| Pronunciation | US: /ˈkwaɪnaɪn/, /kwɪˈniːn/ or UK: /ˈkwɪniːn/ KWIN-een |
| Trade names | Qualaquin, Quinate, Quinbisul, others |
| |
| Clinical data | |
| Main uses | Malaria[1] |
| Side effects | Headache, ringing in the ears, trouble seeing, sweating[2] |
| Pregnancy category |
|
| Routes of use | By mouth, intramuscular, intravenous, rectal |
| Defined daily dose | 1.5 g (by mouth or injection)[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682322 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 70–95%[4] |
| Metabolism | Liver (mostly CYP3A4 and CYP2C19-mediated) |
| Elimination half-life | 8–14 hours (adults), 6–12 hours (children)[4] |
| Excretion | Kidney (20%) |
| Chemical and physical data | |
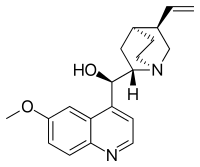
| Formula | C20H24N2O2 |
| Molar mass | 324.424 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 177 °C (351 °F) |
| |
| |
Quinine is a medication used to treat malaria and babesiosis.[2] This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available.[2][5] While sometimes used for restless legs syndrome, quinine is not recommended for this purpose due to the risk of serious side effects.[2] It can be taken by mouth or intravenously.[2] Malaria resistance to quinine occurs in certain areas of the world.[2] Quinine is also the ingredient in tonic water that gives it its bitter taste.[6]
Common side effects include headache, ringing in the ears, trouble seeing, and sweating.[2] More severe side effects include deafness, low blood platelets, and an irregular heartbeat.[2] Use can make one more prone to sunburn.[2] While it is unclear if use during pregnancy causes harm to the baby, treating malaria during pregnancy with quinine when appropriate is still recommended.[2] Quinine is an alkaloid, a naturally occurring chemical compound.[2] How it works as a medicine is not entirely clear.[2]
Quinine was first isolated in 1820 from the bark of a cinchona tree, which is native to Peru.[2][7][8] Bark extracts had been used to treat malaria since at least 1632 and it was introduced to Spain as early as 1636 by Jesuit missionaries from the New World.[9] Quinine is on the World Health Organization's List of Essential Medicines.[10] The wholesale price in the developing world is about US$1.70 to $3.40 per course of treatment.[11] In the United States a course of treatment costs more than $200.[12]
Medical uses[edit | edit source]
As of 2006, quinine is no longer recommended by the World Health Organization (WHO) as a first-line treatment for malaria, because there are other substances that are equally effective with fewer side effects. They recommend that it be used only when artemisinins are not available.[13][14][15][16]
Quinine was frequently prescribed as an off-label treatment for leg cramps at night, but this has become less common due to a warning from the US Food and Drug Administration (FDA) that such practice is associated with life-threatening side effects.[17][18][19]
Dosage[edit | edit source]
The defined daily dose is 1.5 g (by mouth or by injection).[3] The dose for malaria in those who weigh more than 50 kg is 600 mg three times per day for a week.[1] In those less than 50 kg the dose is 10 mg/kg three times per day for a week.[1] The dose is the same for the sulfate, hydrochloride, and dihydrochloride salts of quinine.[1] While, 10 mg of these salts equals 14 mg of quinine bisulfate.[1]
Contraindications[edit | edit source]
Because of the narrow difference between its therapeutic and toxic effects, quinine is a common cause of drug-induced disorders, including thrombocytopenia and thrombotic microangiopathy.[20] Even from minor levels occurring in common beverages, quinine can have severe adverse effects involving multiple organ systems, among which are immune system effects and fever, hypotension, hemolytic anemia, acute kidney injury, liver toxicity, and blindness.[20] In people with atrial fibrillation, conduction defects, or heart block, quinine can cause heart arrhythmias, and should be avoided.[21]
Quinine can cause hemolysis in G6PD deficiency (an inherited deficiency), but this risk is small and the physician should not hesitate to use quinine in people with G6PD deficiency when there is no alternative.[22]
Side effects[edit | edit source]
Quinine can cause unpredictable serious and life-threatening blood and cardiovascular reactions including low platelet count and hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP), long QT syndrome and other serious cardiac arrhythmias including torsades de pointes, blackwater fever, disseminated intravascular coagulation, leukopenia, and neutropenia.[2] Some people who have developed TTP due to quinine have gone on to develop kidney failure.[2][22] It can also cause serious hypersensitivity reactions include anaphylactic shock, urticaria, serious skin rashes, including Stevens–Johnson syndrome and toxic epidermal necrolysis, angioedema, facial edema, bronchospasm, granulomatous hepatitis, and itchiness.[2][22]
The most common adverse effects involve a group of symptoms called cinchonism, which can include headache, vasodilation and sweating, nausea, tinnitus, hearing impairment, vertigo or dizziness, blurred vision, and disturbance in color perception.[2][20][22] More severe cinchonism includes vomiting, diarrhea, abdominal pain, deafness, blindness, and disturbances in heart rhythms.[22] Cinchonism is much less common when quinine is given by mouth, but oral quinine is not well tolerated (quinine is exceedingly bitter and many people will vomit after ingesting quinine tablets).[2] Other drugs, such as Fansidar (sulfadoxine with pyrimethamine) or Malarone (proguanil with atovaquone), are often used when oral therapy is required. Quinine ethyl carbonate is tasteless and odourless,[23] but is available commercially only in Japan. Blood glucose, electrolyte and cardiac monitoring are not necessary when quinine is given by mouth.
Quinine has diverse unwanted interactions with numerous prescription drugs, such as potentiating the anticoagulant effects of warfarin.[2]
Mechanism of action[edit | edit source]
Quinine is used for its toxicity to the malarial pathogen, Plasmodium falciparum, by interfering with the parasite's ability to dissolve and metabolize hemoglobin.[2][24] As with other quinoline antimalarial drugs, the precise mechanism of action of quinine has not been fully resolved, although in vitro studies indicate it inhibits nucleic acid and protein synthesis, and inhibits glycolysis in P. falciparum.[2] The most widely accepted hypothesis of its action is based on the well-studied and closely related quinoline drug, chloroquine. This model involves the inhibition of hemozoin biocrystallization in the heme detoxification pathway, which facilitates the aggregation of cytotoxic heme.[medical citation needed] Free cytotoxic heme accumulates in the parasites, causing their deaths.[25] Quinine may target the malaria purine nucleoside phosphorylase enzyme.[26]
Chemistry[edit | edit source]
The UV absorption of quinine peaks around 350 nm (in UVA). Fluorescent emission peaks at around 460 nm (bright blue/cyan hue).[27] Quinine is highly fluorescent (quantum yield ~0.58) in 0.1 M sulfuric acid solution.[28][29]
Biosynthesis[edit | edit source]
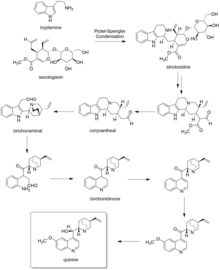
In the first step of quinine biosynthesis, the enzyme strictosidine synthase catalyzes a stereoselective Pictet–Spengler reaction between tryptamine and secologanin to yield strictosidine.[30][31]
Suitable modification of strictosidine leads to an aldehyde. Hydrolysis and decarboxylation would initially remove one carbon from the iridoid portion and produce corynantheal. Then the tryptamine side-chain were cleaved adjacent to the nitrogen, and this nitrogen was then bonded to the acetaldehyde function to yield cinchonaminal. Ring opening in the indole heterocyclic ring could generate new amine and keto functions. The new quinoline heterocycle would then be formed by combining this amine with the aldehyde produced in the tryptamine side-chain cleavage, giving cinchonidinone. For the last step, hydroxylation and methylation gives quinine.[32][33]
Synthesis[edit | edit source]
Cinchona trees remain the only economically practical source of quinine. However, under wartime pressure during World War II, research towards its synthetic production was undertaken. A formal chemical synthesis was accomplished in 1944 by American chemists R.B. Woodward and W.E. Doering.[34]
Since then, several more efficient quinine total syntheses have been achieved,[35] but none of them can compete in economic terms with isolation of the alkaloid from natural sources. The first synthetic organic dye, mauveine, was discovered by William Henry Perkin in 1856 while he was attempting to synthesize quinine.
History[edit | edit source]
Quinine was used as a muscle relaxant by the Quechua people, who are indigenous to Peru, Bolivia and Ecuador, to halt shivering due to low temperatures.[36] The Quechua would mix the ground bark of cinchona trees with sweetened water to offset the bark's bitter taste, thus producing something similar to tonic water.[37]
Spanish Jesuit missionaries were the first to bring cinchona to Europe. The Spanish had observed the Quechua's use of cinchona and were aware of the medicinal properties of cinchona bark by the 1570s or earlier: Nicolás Monardes (1571) and Juan Fragoso (1572) both described a tree, which was subsequently identified as the cinchona tree, whose bark was used to produce a drink to treat diarrhea.[38] Quinine has been used in unextracted form by Europeans since at least the early 17th century.[39]
It was first used to treat malaria in Rome in 1631. A popular story of how it was brought to Europe by the Countess of Chinchon was debunked by medical historian Alec Haggis around 1941.[40] During the 17th century, malaria was endemic to the swamps and marshes surrounding the city of Rome. It had caused the deaths of several popes, many cardinals and countless common Roman citizens. Most of the Catholic priests trained in Rome had seen malaria victims and were familiar with the shivering brought on by the febrile phase of the disease.
The Jesuit brother Agostino Salumbrino (1564–1642),[41] an apothecary by training who lived in Lima (now in present-day Peru), observed the Quechua using the bark of the cinchona tree to treat such shivering. While its effect in treating malaria (and malaria-induced shivering) was unrelated to its effect in controlling shivering from rigors, it was a successful medicine against malaria. At the first opportunity, Salumbrino sent a small quantity to Rome for testing as a malaria treatment.[42] In the years that followed, cinchona bark, known as Jesuit's bark or Peruvian bark, became one of the most valuable commodities shipped from Peru to Europe. When King Charles II was cured of malaria at the end of the 17th Century with quinine, it became popular in London.[43] It remained the antimalarial drug of choice until the 1940s, when other drugs took over.[44]
The form of quinine most effective in treating malaria was found by Charles Marie de La Condamine in 1737.[45][46] In 1820, French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou first isolated quinine from the bark of a tree in the genus Cinchona – probably Cinchona officinalis – and subsequently named the substance.[47] The name was derived from the original Quechua (Inca) word for the cinchona tree bark, quina or quina-quina, which means "bark of bark" or "holy bark". Prior to 1820, the bark was dried, ground to a fine powder, and mixed into a liquid (commonly wine) in order to be drunk. Large-scale use of quinine as a malaria prophylaxis started around 1850. In 1853 Paul Briquet published a brief history and discussion of the literature on "quinquina".[48]
Quinine played a significant role in the colonization of Africa by Europeans. The availability of quinine for treatment had been said to be the prime reason Africa ceased to be known as the "white man's grave". A historian said, "it was quinine's efficacy that gave colonists fresh opportunities to swarm into the Gold Coast, Nigeria and other parts of west Africa".[49]
To maintain their monopoly on cinchona bark, Peru and surrounding countries began outlawing the export of cinchona seeds and saplings in the early 19th century. The Dutch government persisted in its attempts to smuggle the seeds, and by the late 19th century the Dutch grew the plants in Indonesian plantations. Soon they became the main suppliers of the tree. In 1913 they set up the Kina Bureau, a cartel of cinchona producers charged with controlling price and production.[50] By the 1930s Dutch plantations in Java were producing 22 million pounds of cinchona bark, or 97% of the world's quinine production.[49] U.S. attempts to prosecute the Kina Bureau proved unsuccessful.[50]
During World War II, Allied powers were cut off from their supply of quinine when Germany conquered the Netherlands, and Japan controlled the Philippines and Indonesia. The US had obtained four million cinchona seeds from the Philippines and began operating cinchona plantations in Costa Rica. Additionally, they began harvesting wild cinchona bark during the Cinchona Missions. Such supplies came too late. Tens of thousands of US troops in Africa and the South Pacific died of malaria due to the lack of quinine.[49] Despite controlling the supply, the Japanese did not make effective use of quinine, and thousands of Japanese troops in the southwest Pacific died as a result.[51][52][53][54]
Quinine remained the antimalarial drug of choice until after World War II. Since then, other drugs that have fewer side effects, such as chloroquine, have largely replaced it.[55]
Bromo Quinine were brand name cold tablets containing quinine, manufactured by Grove Laboratories. They were first marketed in 1889 and available until at least the 1960s.[56]
Conducting research in central Missouri, Dr. John S. Sappington independently developed an anti-malaria pill from quinine. Sappington began importing cinchona bark from Peru in 1820. In 1832, using quinine derived from the cinchona bark, Sappington developed a pill to treat a variety of fevers, such as scarlet fever, yellow fever, and influenza in addition to malaria. These illnesses were widespread in the Missouri and Mississippi valleys. He manufactured and sold "Dr. Sappington's Anti-Fever Pills" across Missouri. Demand became so great that within three years, Dr. Sappington founded a company known as Sappington and Sons to sell his pills nationwide.[57]
Society and culture[edit | edit source]
Cost[edit | edit source]
Quinine is relatively inexpensive in the UK,[58] where 28 tablets of 300mg quinine sulphate costs the NHS around £5.[59]
Available forms[edit | edit source]
Quinine is a basic amine and is usually provided as a salt. Various existing preparations include the hydrochloride, dihydrochloride, sulfate, bisulfate and gluconate. In the United States, quinine sulfate is commercially available in 324-mg tablets under the brand name Qualaquin.
All quinine salts may be given orally or intravenously (IV); quinine gluconate may also be given intramuscularly (IM) or rectally (PR).[60][61] The main problem with the rectal route is that the dose can be expelled before it is completely absorbed; in practice, this is corrected by giving a further half dose. No injectable preparation of quinine is licensed in the US; quinidine is used instead.[62][63]
| Name | Quinine base equivalence |
|---|---|
| Quinine base | 100 mg |
| Quinine bisulfate | 169 mg |
| Quinine dihydrochloride | 122 mg |
| Quinine gluconate | 160 mg |
| Quinine hydrochloride | 111 mg |
| Quinine sulfate dihydrate [(quinine)2H2SO4∙2H2O] | 121 mg |
Beverages[edit | edit source]

Quinine is a flavor component of tonic water and bitter lemon drink mixers. On the soda gun behind many bars, tonic water is designated by the letter "Q" representing quinine.[64]
According to tradition, because of the bitter taste of anti-malarial quinine tonic, British colonials in India mixed it with gin to make it more palatable, thus creating the gin and tonic cocktail, which is still popular today.[65]
In France, quinine is an ingredient of an apéritif known as quinquina, or "Cap Corse," and the wine-based apéritif Dubonnet. In Spain, quinine (also known as "Peruvian bark" for its origin from the native cinchona tree) is sometimes blended into sweet Malaga wine, which is then called "Malaga Quina". In Italy, the traditional flavoured wine Barolo Chinato is infused with quinine and local herbs, and is served as a digestif. In Canada and Italy, quinine is an ingredient in the carbonated chinotto beverages Brio and San Pellegrino. In Scotland, the company A.G. Barr uses quinine as an ingredient in the carbonated and caffeinated beverage Irn-Bru. In Uruguay and Argentina, quinine is an ingredient of a PepsiCo tonic water named Paso de los Toros. In Denmark, it is used as an ingredient in the carbonated sports drink Faxe Kondi made by Royal Unibrew.
As a flavouring agent in drinks, quinine is limited to less than 83 parts per million in the United States, and 100 mg⁄l in the European Union.[66][67][68]
Scientific uses[edit | edit source]
Quinine (and quinidine) are used as the chiral moiety for the ligands used in Sharpless asymmetric dihydroxylation as well as for numerous other chiral catalyst backbones. Because of its relatively constant and well-known fluorescence quantum yield, quinine is used in photochemistry as a common fluorescence standard.[28][29]
Natural occurrence[edit | edit source]
The bark of Remijia contains 0.5–2% of quinine. The bark is cheaper than bark of Cinchona. As it has an intense taste, it is used for making tonic water.[69]
Regulation in the US[edit | edit source]
From 1969, to 1992, the US Food and Drug Administration (FDA) received 157 reports of health problems related to quinine use, including 23 which had resulted in death.[70] In 1994, the FDA banned the marketing of over-the-counter quinine as a treatment for nocturnal leg cramps. Pfizer Pharmaceuticals had been selling the brand name Legatrin for this purpose. Also sold as a Softgel (by SmithKlineBeecham) as Q-vel[citation needed]. Doctors may still prescribe quinine, but the FDA has ordered firms to stop marketing unapproved drug products containing quinine. The FDA is also cautioning consumers about off-label use of quinine to treat leg cramps.[17][18] Quinine is approved for treatment of malaria, but was also commonly prescribed to treat leg cramps and similar conditions. Because malaria is life-threatening, the risks associated with quinine use are considered acceptable when used to treat that affliction.[71]
Though Legatrin was banned by the FDA for the treatment of leg cramps, the drug manufacturer URL Mutual has branded a quinine-containing drug named Qualaquin. It is marketed as a treatment for malaria and is sold in the United States only by prescription. In 2004, the CDC reported only 1,347 confirmed cases of malaria in the United States.[72]
Cutting agent[edit | edit source]
Quinine is sometimes detected as a cutting agent in street drugs such as cocaine and heroin.[73]
Other animals[edit | edit source]
Quinine is used as a treatment for Cryptocaryon irritans (commonly referred to as white spot, crypto or marine ich) infection of marine aquarium fish.[74]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 "QUININE oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 24 August 2020.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 "Quinine sulfate". Drugs.com. 2020-02-20. Archived from the original on 2015-12-05. Retrieved 2020-05-14.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 22 September 2020.
- ↑ 4.0 4.1 "Qualaquin (quinine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 February 2014. Retrieved 29 January 2014.
- ↑ Esu EB, Effa EE, Opie ON, Meremikwu MM (June 2019). "Artemether for severe malaria". The Cochrane Database of Systematic Reviews. 6: CD010678. doi:10.1002/14651858.CD010678.pub3. PMC 6580442. PMID 31210357.
- ↑ Olmsted, John; Williams, Gregory M. (1997). Chemistry: The Molecular Science. Jones & Bartlett Learning. p. 137. ISBN 978-0-815-18450-8. Archived from the original on 15 September 2016.
- ↑ Willcox, Merlin (28 June 2004). Traditional Medicinal Plants and Malaria. CRC Press. p. 231. ISBN 9780203502327. Archived from the original on 24 February 2020. Retrieved 11 November 2016.
- ↑ Cechinel-Filho, Valdir (2012). Plant bioactives and drug discovery : principles, practice, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 2. ISBN 9780470582268. Archived from the original on 4 March 2016.
- ↑ Staines, Henry M.; Krishna, Sanjeev (2011). Treatment and Prevention of Malaria : Antimalarial Drug Chemistry, Action and Use. [S.l.]: Springer Verlag. p. 45. ISBN 9783034604796. Archived from the original on 2020-02-22. Retrieved 2017-09-10.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Quinine Sulfate". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 12 January 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 47. ISBN 9781284057560.
- ↑ World Health Organization (2006). "Guidelines for the treatment of malaria" (PDF). World Health Organization. Archived from the original (PDF) on 5 August 2009. Retrieved 10 August 2009.
- ↑ Dondorp A, Nosten F, Stepniewska K, Day N, White N (2005). "Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial". Lancet. 366 (9487): 717–25. doi:10.1016/S0140-6736(05)67176-0. PMID 16125588.
- ↑ Reyburn H, Mtove G, Hendriksen I, von Seidlein L (July 2009). "Oral quinine for the treatment of uncomplicated malaria" (PDF). BMJ. 339: b2066. doi:10.1136/bmj.b2066. PMID 19622550. Archived (PDF) from the original on 2018-07-19. Retrieved 2019-02-02.
- ↑ Achan J, Tibenderana JK, Kyabayinze D, Wabwire Mangen F, Kamya MR, Dorsey G, D'Alessandro U, Rosenthal PJ, Talisuna AO (July 2009). "Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial". BMJ. 339: b2763. doi:10.1136/bmj.b2763. PMC 2714631. PMID 19622553.
- ↑ 17.0 17.1 "FDA Drug Safety Communication: New risk management plan and patient Medication Guide for Qualaquin (quinine sulfate)". U.S. Food and Drug Administration (FDA). 7 August 2010. Archived from the original on 19 February 2011. Retrieved 21 February 2011.
- ↑ 18.0 18.1 "Serious risks associated with using Quinine to prevent or treat nocturnal leg cramps (September 2012)". U.S. Food and Drug Administration (FDA). 2012-08-31. Archived from the original on 22 October 2016. Retrieved 19 January 2020.
- ↑ "Quinine for Night-Time Leg Cramps". Consumer Reports. Archived from the original on 2020-04-24. Retrieved 2020-01-20.
- ↑ 20.0 20.1 20.2 Liles NW, Page EE, Liles AL, Vesely SK, Raskob GE, George JN (May 2016). "Diversity and severity of adverse reactions to quinine: A systematic review". American Journal of Hematology. 91 (5): 461–6. doi:10.1002/ajh.24314. PMID 26822544.
- ↑ "Off-label use of sildenafil in valvular heart disease should be avoided". Clinical Pharmacist. 2017. doi:10.1211/cp.2017.20203778. ISSN 2053-6178. Archived from the original on 2021-08-29. Retrieved 2020-05-22.
- ↑ 22.0 22.1 22.2 22.3 22.4 "US label: quinine sulfate" (PDF). FDA. April 2013. Archived (PDF) from the original on 20 January 2017.
- ↑ Jamaludin A, Mohamad M, Navaratnam V, Selliah K, Tan SC, Wernsdorfer WH, Yuen KH (February 1988). "Relative bioavailability of the hydrochloride, sulphate and ethyl carbonate salts of quinine". British Journal of Clinical Pharmacology. 25 (2): 261–3. doi:10.1111/j.1365-2125.1988.tb03299.x. PMC 1386482. PMID 3358888.
- ↑ DrugBank, ed. (24 February 2017). "Quinine". DrugBank. Archived from the original on 6 February 2017.
- ↑ Foley M, Tilley L (February 1997). "Quinoline antimalarials: mechanisms of action and resistance". International Journal for Parasitology. 27 (2): 231–40. doi:10.1016/s0020-7519(96)00152-x. PMID 9088993.
- ↑ Lowe, Derek (22 January 2019). "Quinine's Target". Science. Archived from the original on 29 January 2019. Retrieved 28 January 2019.
- ↑ "Basic Concepts in Fluorescence". Archived from the original on 13 September 2012.
- ↑ 28.0 28.1 Joseph R. Lakowicz. Principles of Fluorescence Spectroscopy Archived 1 August 2016 at the Wayback Machine 3rd edition. Springer (2006). ISBN 978-0387-31278-1. Chapter 2. page 54.
- ↑ 29.0 29.1 Quinine sulfate Archived 10 November 2013 at the Wayback Machine ogi.edu. Retrieved 16 August 2013
- ↑ Treimer JF, Zenk MH (November 1979). "Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation". European Journal of Biochemistry. 101 (1): 225–33. doi:10.1111/j.1432-1033.1979.tb04235.x. PMID 510306.
- ↑ Mizukami H, Nordlöv H, Lee SL, Scott AI (August 1979). "Purification and properties of strictosidine synthetase (an enzyme condensing tryptamine and secologanin) from Catharanthus roseus cultured cells". Biochemistry. 18 (17): 3760–3. doi:10.1021/bi00584a018. PMID 476085.
- ↑ Medicinal natural products : a biosynthetic approach (3rdition ed.). Wiley. pp. 380–381. ISBN 9780470742761.
- ↑ O'Connor SE, Maresh JJ (August 2006). "Chemistry and biology of monoterpene indole alkaloid biosynthesis". Natural Product Reports. 23 (4): 532–47. doi:10.1039/b512615k. PMID 16874388.
- ↑ Woodward R, Doering W (1944). "The Total Synthesis of Quinine". J Am Chem Soc. 66 (849): 849. doi:10.1021/ja01233a516.
- ↑ Kaufman, Teodoro S.; Rúveda, Edmundo A. (2005). "Die Jagd auf Chinin: Etappenerfolge und Gesamtsiege". Angewandte Chemie International Edition (in Deutsch). 117 (6): 876–907. doi:10.1002/ange.200400663.
- ↑ "History of quinine:" Friedrich A. Flückiger and Daniel Hanbury, Pharmacographia: A history of the principal drugs of vegetable origin, met with in Great Britain and British India (London, England: Macmillan and Co., 1874), pages 302-331: Cortex Cinchonæ Archived 11 November 2014 at the Wayback Machine.
- ↑ Hobbs, Kevin; West, David (2020). The Story of Trees : and how they changed the way we live. illustrated by Thibaud Hérem. London: Laurence King. p. 148. ISBN 978-1-7862-7522-6.
- ↑ See:
- Fernando I. Ortiz Crespo (1995) "Fragoso, Monardes and pre-Chinchonian knowledge of Cinchona," Archives of Natural History, 22 (2) : 169–181.
- David C. Stuart, Dangerous Garden: The Quest for Plants to Change Our Lives (London, England: Frances Lincoln Ltd., 2004), p. 28. Archived 4 June 2016 at the Wayback Machine
- Nicolás Monardes, Primera, segunda y tercera partes de la Historia Medicinal de las cosas que le traen de nuestras Indias Occidentales y que sirven en Medicina [First, second and third parts of the medical history of things that have been brought from the new West Indies and that are of use in medicine] (Seville, Spain: Fernando Diaz, 1580), pp. 74-75. Archived 8 May 2016 at the Wayback Machine From p. 74: "Del nuevo Reyno, traen una corteza, que dizen ser de un arbol, que es de mucha grandeza: el qual dize, que lleva unas hojas de forma de coraçon, y que no lleva fruto. Este arbol tiene una corteza gruessa, muy solida y dura, que en esto y en el color parece mucho a la corteza del palo que llaman Guayacan: en la superfiecie tiene una pelicula delgada blanquisca, quebrada por toda ella: tiene la corteza mas de un dedo de gruesso, solida, y pesada: la qual gustada tiene notable amargor, como el dela Genciana: tiene en el gusto notable astriction, con alguna aromaticidad, porque al fin del mascar la respira della buen olor. Tienen los Indios esta corteza en mucho, y usan de lla en todo genero de camaras, que sean con sangre, o sin ella. Los Españoles fatigados de aquesta enfermedad, por aviso de los Indios, han usado de aquesta corteza y han sanado muchos del los con ella. Toman della tanto como una hava pequeña hecha poluos, toma se en vino tinto, o en agua apropiada, como tienen la calentura, o mal: ha se de tomar por la mañana en ayunas, tres o quatro vezes: usando en lo demas, la orden y regimiento que conviene a los que tienen camaras." (From the new kingdom, there is brought a bark, which is said to be from a tree, which is very large: it is said that it bears leaves in the form of a heart, and that it bears no fruit. This tree has a thick bark, very solid and hard, that in this and in its color looks much like the bark of the tree that is called guayacán [i.e., lignum vitae]: on the surface, it has a thin, discontinuous whitish film throughout it: it has bark more than one finger thick, solid and heavy: which, when tasted, has a considerable bitterness, like that of the gentian: it has in its taste a considerable astringency, with some aromaticity, because at the end of chewing it, one breathes with a sweet odor. The Indians hold this bark in high regard, and use it for all sorts of diarrhea, that are with blood [i.e., bloody] and without it. The Spanish [who are] tired of this disease, on the advice of the Indians, have used this bark and have healed many of those with it. They take as much as a small bean, make [it into] powder, take it in red wine or in appropriate water, if they have fever or illness: it must be taken in the morning on an empty stomach, three or four times: otherwise, using the order and regimen that suits those who have diarrhea.)
- Fragoso, Juan, Discursos de las cosas Aromáticas, árboles y frutales, y de otras muchas medicinas simples que se traen de la India y Oriental y sirven al uso de la medicina [Discourse on fragrant things, trees and fruits, as well as many other ordinary medicines that have been brought from India and the Orient and are of use to medicine] (Madrid, Spain: Francisco Sanchez, 1572), p. 35. Archived 5 May 2016 at the Wayback Machine From p. 35: "En el nuevo mundo ay un grande arbol que lleva las hojas a forma de coraçon, y carece de fruto. Tiene dos cortezas, la una gruessa muy solida dura, que assi en la sustancia como en el color es muy semejante al Guayacan: la otra es mas delgada y blaquezina, la qual es amarga con alguna estipticidad: y de mas desto es aromatica. Tienen la en mucho nuestros Indios, porque la usan contra qualesquier camaras, tomando de poluo peso de uno drama o poco mas, desatado en agua azerada, o vino tinto." (In the new world, there is a big tree that bears leaves in the form of a heart, and lacks fruit. It has two barks, one [is] thick, very solid, [and] hard, which in substance as well as in color is much like guayacan [i.e., lignum vitae]: the other is thinner and whitish, which is bitter with some styptic [i.e., astringent] quality: and besides this, it is aromatic. Our Indians regard it highly, because they use it against any diarrheas, taking a weight of a dram or a bit more of the powder, mixing it in mineral water, or red wine.)
- ↑ Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D'Alessandro U (May 2011). "Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria". Malaria Journal. 10: 144. doi:10.1186/1475-2875-10-144. PMC 3121651. PMID 21609473.
- ↑ Stephanie Pain (Sep 15, 2001). "The Countess and the cure". New Scientist. Archived from the original on December 20, 2019. Retrieved December 20, 2019.
- ↑ Juan Eusebio Nieremberg and Alonso de Andrade, Varones Ilustres en Santidad, Letras, y Zelo de las Almas. De la Compañia de Jesus. [Illustrious men in holiness, letters, and zeal for souls. Of the Society of Jesus (i.e., Jesuits).] (Madrid, Spain: Joseph Fernandez de Buendia, 1666), vol. 5, Vida del devoto Hermano Agustin Salumbrino (The life of the devout Brother Agustin Salumbrino), pp. 612–628 ; see p. 612. Archived 22 May 2016 at the Wayback Machine From p. 612: "Nacio el Hermano Agustin Salumbrino el año de mil y quinientos y sesenta y quatro en la Ciudad de Flori en le Romania, … " (Brother Agustino Salumbrino was born in the year 1564 in the city of Flori [Note: This is an error; he was born in Forli.] in Emilia-Romagna, … )
- ↑ See:
- Francisco Medina Rodríguez (July 2007) "Precisiones sobre la historia de la quina" Archived 4 March 2016 at the Wayback Machine (Details about the history of quinine), Reumatología Clínica, 3 (4) : 194–196. (in Spanish) From p. 195: "De hecho, aunque no esté dicha la ultima palabra, hay escritos jesuitas que mencionan que la quina llegó a Roma en 1632, con el provincial de las missiones jusuitas del Perú, el padre Alonso Messia Venegas, como su introductor, cuando trajo una muestra de la corteza para presentaria como primicia, quien había partido de Lima 2 años antes, ya que consta que estuvo en Sevilla en 1632, donde publicó uno de sus libros y siguió su camino hacia Roma en calidad de procurador." (In fact, however, it is not the last word: there are Jesuit writings that mention that quinine arrived in Rome in 1632, with the provincial of the Jesuit missions of Peru, Father Alonso Messia Venegas, as its introducer, when he brought a sample of the bark so that it could be presented as a novelty, which had left Lima two years before, since in fact it had been in Seville in 1632, where he published one of his books and [then] he went his way toward Rome in the capacity of procurator.)
- Enrique Torres Saldamando, Los Antiguos Jesuitas del Perú [The old Jesuits of Peru] (Lima, Peru: Imprenta Liberal, 1882), pp. 180-191 ; see especially p. 181. Archived 10 April 2016 at the Wayback Machine (in Spanish) From p. 181: "Al siguiente año se dirigieron á Europa los Procuradores P. Alonso Messía Venegas y P. Hernando de Leon Garavito, llevando gran cantidad de la corteza de la quina, cuyo conocimiento extendieron por el mundo los jesuitas." (In the following year [i.e., 1631] there went to Europe the procurators Father Alonso Messia Venegas and Father Hernando de Leon Garavito, taking a great quantity of cinchona bark, knowledge of which the Jesuits spread throughout the world.)
- Alberto Bailetti, Blog: "La Misión del Jesuita AgustÍn Salumbrino, la malaria y el árbol de quina" Archived 4 March 2016 at the Wayback Machine (The mission of the Jesuit Agustin Salumbrino, malaria and the quinine tree), Chapter 10: La Condensa de Chinchón (The countess of Chinchon). (in Spanish), excerpt: "A últimas horas de la tarde del treinta y uno de mayo de 1631 se hizo a la vela la armada real con dirección a Panamá llevando el millonario cargamento de oro y plata.
- En una de las naves viajaban los procuradores jesuitas padres Alonso Messia y Hernando León Garavito custodiando los fardos con la corteza de quina en polvo, preparados por Salumbrino. Después de casi veinte días de navegación el inapreciable medicamento llegó a la ciudad de Panamá, donde fue descargado para cruzar en mulas el agreste camino del itsmo palúdico hasta Portobelo para seguir a Cartagena y la Habana, cruzar el Atlántico y llegar a Sanlúcar de Barrameda en Sevilla. … Finalmente siguió su camino a Roma y a su destino final el Hospital del Espíritu Santo."
- (Late in the afternoon of the 31st of May, 1631, the royal armada set sail in the direction of Panama, carrying its multimillion [dollar] cargo of gold and silver.
- On one of the ships traveled the Jesuit procurators Fathers Alonso Messia and Hernando León Garavito, guarding the cases of powdered cinchona bark, prepared by Salumbrino. After almost 20 days of sailing, medicine arrived in the city of Panama, where it was transloaded onto mules. It then traveled the malarial isthmus as far as Portobelo, thence to Cartagena [in Colombia] and Havana. It then traveled to Sanlúcar de Barrameda in Seville, [Spain]. … Finally it followed the road to Rome and to its final destination, the Hospital of the Holy Spirit.)
- ↑ Rocco, Fiametta (2004). Quinine: malaria and the quest for a cure that changed the world. New York, NY: Perennial.
- ↑ Loren, Humphrey (2000). Quinine and Quarantine.
- ↑ de la Condamine (1738) "Sur l'arbre du quinquina" Archived 7 May 2016 at the Wayback Machine (On the quinquina tree) Histoire de l'Académie royale des Sciences, pp. 226–243.
- ↑ See also: Joseph de Jussieu, Description de l'arbre à quinquina: mémoire inédit de Joseph de Jussieu (1737) Archived 19 July 2012 at the Wayback Machine (Description of the quinquina tree: unpublished memoir of Joseph de Jussieu (1737)). De Jussieu accompanied de la Condamine on the latter's expedition to Peru.
- ↑ Pelletier PJ, Caventou JB (1820). "Recherches Chimiques sur les Quinquinas" [Continuation: Chemical Research on Quinquinas]. Annales de Chimie et de Physique (in French). Crochard. 15: 337–365. Archived from the original on 2017-01-26. Retrieved 2016-02-07.
{{cite journal}}: CS1 maint: unrecognized language (link) The authors name quinine on page 348: " …, nous avons cru devoir la nommer quinine, pour la distinguer de la cinchonine par un nom qui indique également son origine." ( …, we thought that we should name it "quinine" in order to distinguish it from cinchonine by means of a name that also indicates its origin.) - ↑ Paul Briquet (1853) Traité thérapeutique du quinquina et de ses preparations from Internet Archive
- ↑ 49.0 49.1 49.2 Conner, Clifford D. (2005). A People's History of Science: Miners, Midwives, and 'Low Mechanicks'. New York: Nation Books. pp. 95–96. ISBN 978-1-56025-748-6. Also cites Porter, Roy (1998). The Greatest Benefit to Mankind: A Medical History of Humanity. New York: W. W. Norton. pp. 465–466. ISBN 978-0-393-04634-2.
- ↑ 50.0 50.1 Shah, Sonia (2010). The Fever: How Malaria Has Ruled Humankind for 500,000 Years. Farrar, Straus and Giroux. p. 94.
- ↑ Louis Morton (1953). "29". The Fall of the Philippines. Washington, D.C.: United States Army. p. 524. Archived from the original on 25 May 2017.
- ↑ Alan Hawk. "Remembering the war in New Guinea: Japanese Medical Corps – malaria". Archived from the original on 22 November 2011.
- ↑ Lt. Gen. Leonard D. Heaton, ed. (1963). "8". Preventive Medicine in World War II: Volume VI, Communicable Diseases: Malaria. Washington, D.C.: Department of the Army. pp. 401 and 434. Archived from the original on 29 January 2012.
- ↑ "Notes on Japanese Medical Services". Tactical and Technical Trends (36). 1943. Archived from the original on 14 October 2011.
- ↑ Shah, Sonia (2010). The Fever: How Malaria Has Ruled Humankind for 500,000 Years. Farrar, Straus and Giroux. p. 102.
- ↑ "Medicine: What's Good for a Cold?". Time. 22 February 1960. Archived from the original on 26 July 2010. Retrieved 27 April 2010.
- ↑ "John. S Sappington". Historic Missourians. State Historical Society of Missouri. Archived from the original on 2020-04-11. Retrieved 2020-04-11.
- ↑ Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. pp. 196–197. ISBN 978-0-7020-7442-4. Archived from the original on 2021-05-22. Retrieved 2021-11-09.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 655-656. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ Barennes H, Pussard E, Mahaman Sani A, Clavier F, Kahiatani F, Granic G, Henzel D, Ravinet L, Verdier F (May 1996). "Efficacy and pharmacokinetics of a new intrarectal quinine formulation in children with Plasmodium falciparum malaria". British Journal of Clinical Pharmacology. 41 (5): 389–95. doi:10.1046/j.1365-2125.1996.03246.x. PMC 2042609. PMID 8735679.
- ↑ Barennes H, Balima-Koussoubé T, Nagot N, Charpentier JC, Pussard E (May 2006). "Safety and efficacy of rectal compared with intramuscular quinine for the early treatment of moderately severe malaria in children: randomised clinical trial". BMJ. 332 (7549): 1055–9. doi:10.1136/bmj.332.7549.1055. PMC 1458599. PMID 16675812.
- ↑ Centers for Disease Control and Prevention (April 1991). "Treatment with quinidine gluconate of persons with severe Plasmodium falciparum infection: discontinuation of parenteral quinine from CDC Drug Service". MMWR. Recommendations and Reports. 40 (RR-4): 21–3. PMID 1850497. Archived from the original on 2018-12-15. Retrieved 2017-09-10.
- ↑ Magill A, Panosian C (July 2005). "Making antimalarial agents available in the United States". The New England Journal of Medicine. 353 (4): 335–7. doi:10.1056/NEJMp058167. PMID 16000347.
- ↑ Charming, Cheryl (2006). Miss Charming's Guide for Hip Bartenders and Wayout Wannabes. USA: Sourcebooks, Inc. p. 189. ISBN 978-1-4022-0804-1.
- ↑ "Gin and Tonic: The fascinating story behind the invention of the classic English cocktail". India.com. 17 March 2017. Archived from the original on 8 June 2019. Retrieved 8 June 2019.
- ↑ Ballestero JA, Plazas PV, Kracun S, Gómez-Casati ME, Taranda J, Rothlin CV, Katz E, Millar NS, Elgoyhen AB (September 2005). "Effects of quinine, quinidine, and chloroquine on alpha9alpha10 nicotinic cholinergic receptors". Molecular Pharmacology. 68 (3): 822–9. doi:10.1124/mol.105.014431. PMID 15955868.
{{cite journal}}: Unknown parameter|displayauthors=ignored (help) - ↑ "Food Additive Status List". U.S. Food and Drug Administration. U.S. Department of Health and Human Services. Archived from the original on 4 November 2017. Retrieved 9 October 2017.
- ↑ "COMMISSION IMPLEMENTING REGULATION (EU) No 872/2012". EUR-Lex. Official Journal of the European Union. Archived from the original on 9 October 2017. Retrieved 9 October 2017.
- ↑ Hobhouse, Henry (2004). Šest rostlin, které změnily svět (in Czech). Prague: Akademie věd České republiky. p. 59. ISBN 978-80-200-1179-4.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ "FDA Orders Stop to Marketing Of Quinine for Night Leg Cramps". FDA Consumer Magazine. U.S. Food and Drug Administration (FDA). July–August 1995. Archived from the original on 2008-01-15. Retrieved 2009-07-31.
- ↑ "FDA Orders Unapproved Quinine Drugs from the Market and Cautions Consumers About Off-Label Use of Quinine to Treat Leg Cramps" (Press release). U.S. Food and Drug Administration (FDA). 11 December 2006. Archived from the original on 28 July 2009. Retrieved 31 July 2009.
- ↑ Skarbinski J, James EM, Causer LM, Barber AM, Mali S, Nguyen-Dinh P, Roberts JM, Parise ME, Slutsker L, Newman RD (May 2006). "Malaria surveillance--United States, 2004" (PDF). MMWR Surveill Summ. 55 (SS-4): 23–37. PMID 16723971. Archived (PDF) from the original on 2020-08-06. Retrieved 2020-01-20.
- ↑ "Microgram Bulletin, DIMETHYLTRYPTAMINE AND ECSTASY MIMIC TABLETS (ACTUALLY CONTAINING 5-METHOXY-METHYLISOPROPYLTRYPTAMINE) IN OREGON" (PDF). U.S. Department of Justice. Drug Enforcement Administration. October 2009. p. 79. Archived from the original (PDF) on October 17, 2012. Retrieved September 22, 2012.
- ↑ Porritt, Michael. "Cryptocaryon irritans". Reef Culture Magazine (1 ed.). Archived from the original on October 24, 2009. Retrieved July 9, 2009.
Further reading[edit | edit source]
- Schroeder-Lein, Glenna (2008). The encyclopedia of Civil War medicine. Armonk, NY: Sharpe, Inc.
- Hobhouse, Henry. Seeds of Change Six Plants that Transformed Mankind. 2005. ISBN 1-59376-049-3.
- Stockwell HR (1982). "Aeromedical considerations of malaria prophylaxis with mefloquine hydrochloride". Aviation, Space, and Environmental Medicine. 3 (10): 1011–13. PMID 6983345.
- Wolff RS, Wirtschafter D, Adkinson C (June 1997). "Ocular quinine toxicity treated with hyperbaric oxygen". Undersea & Hyperbaric Medicine. 24 (2): 131–4. PMID 9171472. Archived from the original on 2011-08-11. Retrieved 2008-08-13.
- Slater, Leo (2009). War and disease : biomedical research on malaria in the twentieth century. New Brunswick, NJ: Rutgers University Press.
- The Lords of Industry Archived 2012-10-19 at the Wayback Machine, Modern History Sourcebook: Henry Demarest Lloyd: North American Review 331 (June 1884)
- World Health Organization (2015). Guidelines for the treatment of malaria, 3rd ed (3rd ed.). World Health Organization (WHO). hdl:10665/162441. ISBN 9789241549127.
External links[edit | edit source]
- Quinine Archived 2007-04-19 at the Wayback Machine at the International Programme on Chemical Safety
| External sites: | |
|---|---|
| Identifiers: |
|