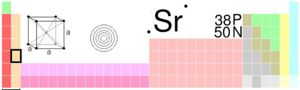
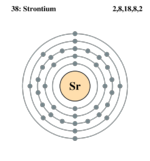
Strontium is the 38th element on the periodic table in Group llA, Period 5. As a member of the alkaline earth metals, strontium is harder than alkali metals like Rubidium (37), and has a higher density. The outer valence shell contains two electrons, causing strontium to be very reactive. For this reason, strontium and similar elements are only found in rock formations rather than in basic, highly reactive elemental forms in the earth's crust. (Cox et al p.120)
History
Strontium was first discovered in 1784 and mistaken for witherite or fluorite. In 1787 and 1790 the first chemical work began on strontium by Crawford and Cruickshank. They determined that strontium contained a new earth (alkaline earth metal), which was confirmed by Thomas Hope in 1791. This newly discovered metal was named strontianite after Scottish village of Strontian. Martin Heinrich Klaproth created stontrium oxide and strontium hydroxide in 1794. It wasn't until 1808 that Sir Humphry Davy isolated metallic strontium through electrolysis. [1]
Properties

A chemical reaction involving strontium chloride.
Strontium is a soft element of a basic silver color in the alkaline earth metal family. When exposed to air, it quickly develops a yellow oxide and spontaneously combusts. It also rapidly decomposes in water. Because of its high reactivity, strontium must be stored away from air or water, preferably under kerosene to prevent the formation of oxide. Strontium occurs naturally as a combination of four stable isotopes. [2] Strontium also exhibits allotropy (occur[ing] in two or more forms in the same physical state) with three crystalline solids. [3]
Occurrences
Strontium is the 15th most abundant element in the earth’s crust at 0.0384% following barium and fluorine. It’s not found naturally isolated, but in mixtures and compounds. [4] Celesite (strontium sulfate) and strontianite (strontium carbonate) are the only two minerals that contain enough strontium to be used for mining purposes. Strontium is also present in 0.034% of all igneous rock. [5] The largest concentrations of celesite in the world are in Mexico, Spain, Turkey, and regions of Russia. Strontium occurrence in sea water is 0.1 mg/l , and is a primary source of strontium, containing billions of tons! Marine protozoa known as radiolarians have skeletons composed almost primarily of a strontium compound. The soil concentration of strontium is 0.035%, and various plants, such as legumes, accumulate strontium. It is estimated that there are 10 9 tons of strontium in deposits all over the earth. [6] Strontium can only be isolated by melting and electrolyzing strontium chloride, which separates the compound into strontium and chlorine gas. [7]
Uses

Strontium is used in fireworks to give off a crimson glow.
Strontium is used in a variety of ways, especially because it has highly reactive. The chief production of strontium is for making glass in the picture tubes of color television. It is also used to create magnets and refine zinc. A soft form of strontium, strontium titanate, has been used as a gemstone because its dispersion of light is greater than a diamond's. [8] Strontium salts are frequently used in pyrotechnics because they give off a crimson color. Other strontium "soaps" are used in the production of different axle-greases, and strontium-90, a radioactive and β-decay isotope, is used as an atomic power source. Strontium is also used in photocell and fluorescent dye production. Metallic strontium forms aid in the deoxidation of bronze and copper, and refines steels from phosphorus and sulfur. Strontium carbonate is found in porcelain enamels and weatherproof glazes, and other strontium compounds are used in corrosion- and rust-resistant pigments of varnishes and paints. [9]
Medicinal Value
During the 1950s and 1960s nuclear testing resulted in fall out radioactive strontium-90. This toxic waste permeated the atmosphere, especially in Northern Europe and the former Soviet Republic. Nuclear reactors and nearby areas were also frequently infected with large amounts of strontium-90. Ingested or inhaled strontium-90 can result in bone cancer or leukemia, although most of the strontium passes through the body. The strontium, which is similar to calcium, tends to concentrate in teeth and bones. [10] During this time, many people were infected with radioactive strontium-90 when it contaminated dairy products and the environment. Today however, strontium is being used in small dosages to help treat and prevent osteoporosis. The human body naturally contains about 320 mg of strontium, and strontium supplements in patients suffering from osteoporosis reduces the pain and increases the bone density. A study under Dr. Stanley C. Skoryna in 1985 revealed in bone biopsies a 172% increase in the rate of bone formation after strontium therapy and a decrease in pain. Administering medicinal strontium can also reduce the number of fractures.
In 1981 Dr. Skoryna treated breast and prostate cancer patients with strontium. These patients’ cancer had moved into their bones, and the effect of strontium within the body was remarkable. New mineral deposits of both calcium and strontium appeared in the bones, and strengthened the weakening bones. Strontium can also prevent cavities when it is in concentrations of 6-10 mg/liter in drinking water. Further studies of medical uses for strontium in Belgium concluded that it has a cartilage-growth-promoting effect that can be useful in many areas including various types of arthritis. The optimum dose of strontium salts (strontium carbonate, strontium lactate, strontium gluconate, etc.) is about 680 mg/day or less. It shouldn’t be taken with calcium supplements however, because the calcium reduces the absorption of strontium. This inexpensive treatment and prevention for bone related health conditions is an important advance in medicine. [11]
As of January 1990, strontium metal (98%) cost $5 an ounce. [12]
References