| Rutherfordium |
 |
| General Info |
| Atomic Symbol |
Atomic symbol::Rf |
| Atomic Number |
Atomic number::104 |
| Atomic Weight |
Atomic weight::261.11 g/mol |
| Chemical series |
Transition metal |
| Appearance |
Unknown, most likely metallic grey in color.
 |
| Group, Period, Block |
4, 7, d |
| Electron configuration |
[Rn] 5f14, 6d2, 7s2 |
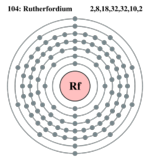
| Electrons per shell |
2 8, 18, 32, 32, 10, 2
 |
| CAS number |
CAS number::53850-36-5 |
| Physical properties |
| Phase |
Solid[1][2] |
| Density |
Density::23 (estimated) g/ml |
| Melting point |
Melting point::(estimated) 2400K |
| Boiling point |
Boiling point::(estimated) 5800K |
| Isotopes of Rutherfordium |
| iso |
NA |
half-life |
DT |
DE (MeV) |
DP |
| 253Rf |
syn |
48 μs |
α-SF |
9.500 |
249No |
| 254Rf |
syn |
22.3 μs |
SF |
9.300 |
250No |
| 255Rf |
syn |
1.6 ms |
α-SF |
9.300 |
251No |
| 256Rf |
syn |
6.2 ms |
α-SF |
8.952 |
252No |
| 257Rf |
syn |
4.7 s |
α-β-SF |
9.250, 3.400 |
253No, 257Lr |
| 258Rf |
syn |
13 ms |
α-SF |
9.250 |
254No |
| 259Rf |
syn |
3.1 s |
α-SF |
9.110 |
255No, 259Lr |
| 260Rf |
syn |
20 ms |
SF-α-β |
9.000, 2.450 |
256No |
| 261Rf |
syn |
70 s |
α-β-SF |
8.810, 1.800 |
257No, 261Lr |
| 262Rf |
syn |
2.1 s |
SF |
|
258No |
| 263Rf |
syn |
15 m |
SF-α |
|
259No |
| 264Rf |
syn |
1 h |
|
|
|
| 265Rf |
syn |
2.5 m |
SF |
|
|
| 266Rf |
syn |
10 h |
|
|
|
| 267Rf |
syn |
1.3 h |
SF |
|
|
| 268Rf |
syn |
6 h |
[3][4] |
|
|
|
| All properties are for STP unless otherwise stated. |
Rutherfordium is a chemical element named after Ernest Rutherford. It is classified as a transitional metal, which can be found as the 104th element on the periodic table. It is so large, in fact, that it is too unstable to exist in nature. It can only be made with particle colliders, and even then it is so radioactive that it quickly decays into smaller elements. Currently it has no uses, but scientists are still studying elements like rutherfordium, hoping to find some practical use for them.[5]
History
In 1964, a group of Russian scientists at the Joint Institute for Nuclear Research in Dubna, Russia first reported creating the element Rutherfordium. They placed plutonium-242 into a particle collider, then bombarded the plutonium with neon-22 atoms.[6] However, they were not able to find its half-life, or even its isotope number. They had merely identified a new, unknown element. Three years later, at the Lawrence Berkeley Laboratory in Berkeley, California, a group of scientists tried to reproduce the Russian experiment. They went with a slightly different method, deciding to fire carbon-12 atoms at californium-249 instead of the neon-plutonium combination used by the Russians. They believed that they had discovered rutherfordium, and a few years later another group confirmed their findings. Since it was unclear whether the Russians or the Americans discovered the element, a small argument broke out over who would get to name the element. The Russians wanted to name it kurchatovium after Igor Vasilyevich Kurchatov, a Russian nuclear physicist who helped work on Russia's first atomic bomb. The Americans wanted to name it rutherfordium after Ernest Rutherford, a scientist who theorized a model for the atom and discovered nuclear fission. The IUPAC eventually decided that the name of the element would be rutherfordium.[5]
How It Is Made
Creating synthetic elements like rutherfordium is a very complicated process. Large devices called particle colliders are used for the creation process. By using magnetic fields, ions are pulled through a long, hollow tube. As they go through the tube, they slowly start to gain speed. The ions need to be travelling incredibly fast in order to overcome the repulsion between the positively-charged nuclei of the colliding atoms. When the ions are travelling fast enough, they collide with larger atoms, and the nuclei of the atoms fuse together. For creating rutherfordium, scientists originally fired neon ions and plutonium ions, but other combinations have worked as well. The type of atoms used for this process depend on the element that is being created.[7]
Properties
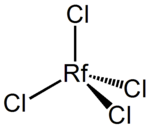
The hypothetical structure of RfCl
4
Since rutherfordium is so radioactive, it is incredibly hard to study. In fact, very little about its specific physical and chemical properties has been recorded. Since elements have similar characteristics to other elements in their groups, it is expected that rutherfordium has characteristics similar to hafnium and zirconium.[5] The appearance of rutherfordium is unknown since it has never been seen, but it is expected that it would be metallic grey in color, like many other transition metals. Also, it would probably be malleable, hard, and able to conduct electricity. Like other transition metals, it would also probably have low ionization energy.[8] Its oxidation number is +4.[6] Rutherfordium's unstable nature has made it difficult to form any compounds with it. However, it has been found that it can form strong chloride complexes. Chemists believe that rutherfordium could bond with chlorine, forming rutherfordium chloride with the formula RfCl4. This compound is believed to have similar qualities to zirconium chloride and hafnium chloride, except rutherfordium chloride would be much more volatile.[5]
Occurrences
Rutherfordium is a very large element. Therefore, it is extremely unstable, and it quickly decays into smaller, different elements. Since it is so unstable and so radioactive, it cannot exist naturally anywhere in the known universe. Rutherfordium can only be created in laboratories by using particle colliders. Only a small amount is made through the creation process, and since it is so radioactive, the small amount that is made does not last long.[6]
Uses
At this point in time, no common use for rutherfordium exists. It is very radioactive, making it dangerous to use without protective gear. Also, it decays very quickly, so it is very difficult to research uses for it. It is very likely that rutherfordium will never be used for anything besides scientific research. Rutherfordium is the first of the trans-actinide elements, so scientists will usually study rutherfordium in order to find out about the properties of elements after the actinide series. Also, by creating elements such as rutherfordium, the process of creating new elements can be understood better.[5]
Ernest Rutherford
Ernest Rutherford, the man that rutherfordium was named after, was born in Nelson, New Zealand on August 30, 1871, the fourth child out of twelve. At age eighteen he went to the University of New Zealand, Wellington. In 1893 he graduated with a M.A. with a double first in mathematics and physical science. He continued research at the college, and he received his B.S. degree the following year. That same year, he received a special science scholarship, enabling him to go to Trinity College in Cambridge, where he became a research student under J.J. Thompson. He spent the next couple of years researching and teaching science and physics at multiple universities.[9]
During his many years of research, Rutherford mainly studied radiation and the atom. He also worked along side with many famous chemists and physicists. Rutherford worked with Frederick Soddy to create the disintegration theory of radiation, which states that radiation is an atomic, not a molecular process. He also studied the alpha particle, and in collaboration with H. Geiger, developed a way to count individual alpha particles. He used this method to measure the alpha particle emissions of radium.[9]
Perhaps Rutherford's most famous contribution to science is his model of the atom. In 1909, Rutherford performed an experiment. He set up a source that fired alpha particles in a straight line. He aimed the alpha particles at a sheet of gold foil, and placed his alpha particle detector on the other side of the foil. According to the atomic model at that time, all of the particles should have gone right through the gold foil and been picked up by the detector. However, this did not happen. Many of the atoms did not travel in a straight line; many were bent of course. Some went off at angles as great as ninety degrees. Some even bounced right back at the source! Rutherford realized that the current model of the atom could not explain this phenomenon, so he devised a new atomic model. According to his model, at the center of each atom was a nucleus formed of protons, and the electrons orbited around this nucleus. Ninety-nine percent of the atom's mass lied in the nucleus, but the nucleus was only about 1/100,000 the size of the actual atom.[10]
Rutherford accomplished much over the course of his career. He wrote many books including Radioactivity in 1904 and The Electrical Structure of Matter in 1926. In 1908, he won the Nobel Prize in Chemistry. He worked alongside many future Nobel Prize winners, including Niels Bohr, Patrick Blackett, J.J. Thompson, and James Chadwick. He was even knighted in 1914. He died on October 19, 1937.[9]
References
- ↑ Author Unknown. [1] www.rsc.org. Web. Accessed November 3, 2011
- ↑ Author Unknown. [2] www.wolframalpha.com. Web. Accessed November 3, 2011
- ↑ Author Unknown. [3]. Isotopes of Rutherfordium. Web. 23 September 2011
- ↑ Author Unknown. [4]. Periodic Table of Elements - Rf - Rutherfordium. EnvironmentalChemistry.com. Web. 1995-2011
- ↑ 5.0 5.1 5.2 5.3 5.4 Author Unknown. [5] www.rsc.org. Web. Accessed November 3, 2011
- ↑ 6.0 6.1 6.2 Gagnon, Steve. [6] Jefferson Lab. Web. Accessed 16 November 2011.
- ↑ Author Unknown. [7]. Particle Accelerators. Web. Accessed 17 November 2011
- ↑ Helmenstine, Anne Marie. [8]. Transition Metals: Properties of Element groups. Web. Accessed 16 November 2011.
- ↑ 9.0 9.1 9.2 Author Unknown. [9]. The Nobel Prize in Chemistry 1908: Ernest Rutherford Biography. Web. Accessed 17 November 2011
- ↑ Author Unknown. [10]. The Rutherford Model. Web. Accessed 17 November 2011.