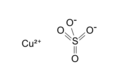
Copper(II) sulfate is a compound with the formula CuO4S. It is formed when a sulfur atom bonded to four oxygen atoms, combines with one copper atom. Copper sulfate has a molar mass of 159.6086 grams per mole. It is soluble in water and has several hydrated forms. Copper sulfate can form naturally in the environment or be produced manually in labs or factories[2]. It has a blue color when hydrated and a pale white color when anhydrous. Copper sulfate can form beautiful blue crystals with a triclinic structure. Copper sulfate can be toxic to the touch, so when handling it use gloves, glasses, and eye wear. Its main use is in agriculture as a pesticide, but it also has several other applications[3].
Properties
Copper sulfate has various properties that make it very useful as well as hazardous. Copper sulfate has a blue color when hydrated and a white color when anhydrous. It also has a pleasing smell. When copper sulfate goes through decomposition, it will break up into cupric acid (CuO) and sulfur trioxide (SO3). The anhydrous form will usually attract water and form copper sulfate pentahydrate in a majority of conditions under thirty degrees Celsius. When it is near clay,copper phosphate will precipitate along with hydroxides and carbonates. Copper sulfate is very reactive with hydrochloric acid. Together they form a green colored tetrachlorocuprate(II). The copper may also be replaced in replacement reactions when more reactive metals are involved[3].
Copper sulfate is not exceptionally dangerous, but it is classified as hazardous. Copper sulfate is very corrosive. For this reason it is not transported in steel and iron or stored in metal capsules. When handling this compound, you must wear protective equipment. The reason being copper sulfate is very irritating to the skin and especially the face. Ingestion of this compound is very dangerous. An amount the size of one gram can cause renal failure or even death. Several symptoms are dizziness, intense abdominal pain and nausea and vomiting[3].
Synthesis / Occurrences
Copper sulfate can form naturally in nature, or be produced by mankind. American Elements copper sulfate facilities manufacture copper sulfate by implementing a process that was created to provide a non-caking, high purity copper sulfate acceptable for both industrial and agricultural applications[4]. The copper in the soil that bonds with the sulfur may originate from natural sources. It can be left behind from pesticides, or other sources. These may include industry, architectural material, mining, and motor vehicles. Copper can accumulate at the surface of the soil, where it then has the chance to bind tightly and persists. Copper sulfate is very soluble in water and it will bind to sediments. Copper is also released by plants. Too much copper is toxic so a plant will release some if it takes in too much[2].
Uses
Copper sulfate has several important uses. The inorganic compound is often used in pesticides. Copper sulfate has been used to kill: bacteria, algae, roots, plants, snails, and fungi. The amount of copper is what contributes to the compounds harmfulness. The way the copper sulfate works, is by first binding to an organism, fungi for example. This will damage the cell of the fungi causing it to leak and eventually die. It also disrupts the normal function of skin cells and enzymes in larger organisms. Copper, however, in proper amounts is an important mineral that is found in our food, water, and other areas of the environment[2].
Along with the compound being useful in agriculture, copper sulfate also has uses in building, as adhesives, and in several other areas. In the area of construction, copper sulfate is very useful. It is an ingredient in concrete, as an antiseptic and to modify how the concrete sets. It is used in wood structures and as a plaster to prevent dry rot. Several other uses include: as a glass coloring, as a printing ink, as an electrolyte, and even in the purification of petroleum oils[5].
Crystal Growing
Copper sulfate is known for forming beautiful blue crystals. For this reason it is common for people to create these crystals on their own, either for an experiment or for simply viewing them. First you will need to acquire some of the compound. You can order it yourself, or create it. To make copper sulfate you will need: copper wire, sulfuric acid, battery acid, water, and a 6-volt battery. First fill a jar with 5 ml concentrated sulfuric acid and 30 ml of water. Set up two copper wires in the solution, but do not let them touch. Then connect the wires to the 6-volt battery. The solution should begin to turn blue as the copper sulfate begins to form. As electricity runs through the wires, the hydrogen gas will bubble off, and the positive electrode will be dissolved into the sulfuric acid and oxidize. You can then boil the formed solution to recover your copper sulfate. The solution does contain sulfuric acid, so you won’t be able to boil away the liquid completely. The copper sulfate will precipitate out of the solution as a blue powder. To get copper sulfate crystals, you can allow them to grow from the solution you have prepared. Be cautious when removing your crystals because the solution is very acidic[6].
Video
This experiment shows the formation of copper sulfate crystals.
References
-sulfate-unit-cell-3D-balls.png)

_sulfate_powder.jpg)