| iso | NA | half-life | DT | DE (MeV) | DP |
| is stable with neutrons. | |||||
| Seaborgium | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||||
| General Info | |||||||||||||||||||
| Atomic Symbol | Atomic symbol::Sg | ||||||||||||||||||
| Atomic Number | Atomic number::106 | ||||||||||||||||||
| Atomic Weight | Atomic weight::263 g/mol | ||||||||||||||||||
| Chemical series | Transition Metals | ||||||||||||||||||
| Appearance | Unknown | ||||||||||||||||||
| Group, Period, Block | 6, 7, d-block | ||||||||||||||||||
| Electron configuration | [Rn] ??, ??, ?? | ||||||||||||||||||
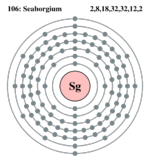
| Electrons per shell | ?, ?  |
||||||||||||||||||
| CAS number | CAS number::54038-81-2 | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Phase | Solid | ||||||||||||||||||
| Density | Density::Not known g/ml | ||||||||||||||||||
| Melting point | Melting point::Not known | ||||||||||||||||||
| Boiling point | Boiling point::Not known | ||||||||||||||||||
| Isotopes of Seaborgium | |||||||||||||||||||
|
|||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||
Seaborgium is a chemical element that can be found as the 106th element in the periodic table. It is radioactive with a very short half-life and currently is not used for anything other than particle collisions. It is part of the transition metals and is assumed to share the qualities of tungsten and molybdenum. Seaborgium is named after Glenn Seaborg, a world renowned chemist who studied radioactivity extensively. [1]
Many properties of seaborgium are unknown because not enough of it has been produced to do testing. It begins to decompose the moment it is formed, with a half-life of less than a second. It is assumed to be a solid even though the density is unknown. Reactivity with other elements or compounds is unknown as well. Seaborgium is assumed to behave similar to tungsten and molybdenum because it fits in the same group as they do. It is a synthetic element and is not found naturally on earth. If seaborgium is in fact a transition metal, it would most likely be a good conductor of heat and electricity. Also if it could be created in a decent amount, it is expected to be fairly malleable.[1]
Seaborgium was created in 1974 at the Lawrence Berkeley Laboratory with a machine known as the SuperHILAC, which is short for Super Heavy Ion Linear Accelerator. The men who were in charge of the collisions were E. Kenneth Hulet and Albert Ghiorso. They caused a collision between californium and oxygen ions to create seaborgium-263 atoms along with some extra neutrons. Seaborgium did not last very long, however. With a half-life of less than a second the scientists did not have very long to study the new and exciting element. Overall, the SuperHILAC discovered five superheavy elements before the machine was retired in 1993.[2]
Since seaborgium has such a short half-life and is extremely radioactive, chemists have had trouble finding any use for Seaborgium.[1]
On April 19, 1912 in Ishpeming, Michigan, the world welcomed a brilliant young chemist. Not at that time of course, but later his accomplishments would include the Nobel Peace Prize, the discovery of many transuranium elements, reorganization of the periodic table, and transmuting lead atoms into gold. He studied chemistry at the University of California Los Angeles where he met Albert Einstein; the experience inspired him to perform chemical research instead of pursuing the career he was thinking of. He then switched colleges and enrolled in the University of California at Berkeley. There he researched radioactivity with a cyclotron. Even though he graduated, he could not resist his passion for chemistry and continued to study chemistry. He helped discover over 100 important isotopes, one of which treated thyroid disease.
In 1942, he married Helen Griggs in Pioche, Nevada, with only two people in attendance. In 1951, he was presented the Nobel Prize in Chemistry for discovering many transuranium elements. At the height of World War 2, Seaborg was vehemently against dropping the atomic bomb on Japan, he wrote a report asking Harry Truman to demonstrate its power before killing thousands without sufficient warning. Throughout his life, Seaborg was appointed president of many different branches of chemical research by United States Presidents such as Harry Truman, John F. Kennedy, Richard Nixon, and Ronald Reagan. In 1980, he succeeded the ultimate alchemy challenge of transmuting lead atoms into gold. However, the process was far too expensive to be of any commercial value.
On August 24, 1998, Glenn Seaborg suffered a large stroke that ultimately lead to his death on February 25, 1999. He has been inducted into the National Inventors Hall of Fame and is one of the most important chemists in American history.[3]
|
||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||