Electronegativity is the measure of an element's ability to attract electrons to itself. This definition of electronegativity was given by Linus Pauling in the 1930's. It is a very important concept in chemistry for several reasons. Electronegativity determines the type of bonds elements will form. Electronegativity also determines the oxidation numbers of elements in a bond. [1]
The idea of electronegativity existed well before Linus Pauling presented his scale of electronegativity. It dates back all the way to the early 1800's. In 1809, an Italian scientist named Amedeo Avogadro published a paper about how bases and acids neutralize each other. The paper also covers how positive and negative charges neutralize each other. He then went on to claim that these concepts of things neutralizing each other could be applied to all reactions. Using this information, Avogadro created a scale which he called the oxygenicity scale. He then used many contact electrification experiments to assign values to the elements on his scale. However, his scale turned out to be inaccurate because measuring contact electricity can be easily affected by outside factors. Later on a Swedish chemist named Jöns Jakob Berzelius improved upon Avogadro's work. He decided to focus more on heat instead of contact electricity and he also used the term electronegativity instead of oxygenicity. His scale turned out to be more accurate than Avogadro's scale but it still was not completely accurate.[2]
In the early 1930's Linus Pauling proposed his version of the electronegativity scale. His scale was based on his observations of how the bonds of similar elements are weaker than the bonds of dissimilar elements. The bonds of dissimilar elements are ionic and Pauling used this information to develop his scale. When fluorine and lithium bond, the bond is almost completely ionic. Pauling then placed these two elements on different sides of his scale. He then assigned values to all the elements using an accepted scale of ionic bond proclivities. Even today, his scale is considered accurate and there has been very little adjustments to it.[3]
When elements bond together their electronegativities determine what kind of bond will form. When elements bond together they share their electrons with each other. Their electronegativities will determine how these electrons are shared. When a metal and a nonmetal bond together the electrons that are shared spend almost all of their time around the nonmetal. This type of bond is called an ionic bond. The electrons spend most of their time around the nonmetal because they have a much higher electronegativity than the metal does. When two elements bond that have similar electronegativities a covalent bond occurs. This happens when two nonmetals bond together. However, even though nonmetals have similar electronegativities the electrons will still spend more time around the more electronegative element. This is called a polar covalent bond. The only way for electrons to be shared equally is if the same element is bonded together. This type of bond is called a nonpolar covalent bond.[4]
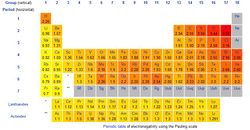
The most common scale used to determine electronegativity is the Pauling scale. In this scale the element with the largest electronegativity is fluorine with a value of 4.0. The element with the lowest electronegativity is francium with a value of 0.7. [1] There are also different scales that can be used to determine electronegativity. Two different scales are the Mulliken scale and the Allred-Rockow scale. The values for the Mulliken scale are mainly determined by electron affinity and ionization energy of the atom. The values for the Allred-Rockow scale are determined by the size and charge of the nucleus. Both of these scales seem to agree with the Pauling scale and they are less commonly used. [5]
Electronegativity is a very important concept in chemistry. In covalent bonds the electronegativity determine the polarity of the bond. Polarity occurs when the electron cloud tends to stay closer to one atom than the other. Electronegativity also plays a very important role in determining the oxidation numbers for elements. Oxidation numbers are how many electrons elements lose or gain in chemical bonding. They are basically the charge of the element and it plays one of the most important roles in chemical bonding.[5]
Oxidation numbers determine the ratio of elements in a compound. There are simple rules to determine the oxidation numbers of all the elements. These rules are all determined by electronegativity. When elements bond the element with the higher electronegativity will have a negative oxidation number and the element with the lower electronegativity will have a positive oxidation number. Two rules are group one elements will always have a +1 oxidation number and group two elements will have a +2 oxidation number. They will always have these oxidation numbers because when they bond to nonmetals they have a lower electronegativity. Another rule is fluorine has a -1 oxidation number. It always has that oxidation number because it has the highest electronegativity. oxygen will also have a -2 oxidation number unless it is bonded to fluorine or in a peroxide ion. [6]
Electronegativity decreases as one goes down a group. This happens as you go down a group because the valence electrons are farther from the nucleus. Valence electrons are the electrons in the outer shell. Electronegativity also increases as one moves across a period. This occurs because there is one more proton as the atomic number rises one. The increased positive charge draws the electrons closer to itself. [7]
Video describing electronegativity.
|
||||||||||||||